Article contents
Hydrothermal synthesis of Mg-substituted tricalcium phosphate nanocrystals
Published online by Cambridge University Press: 28 August 2019
Abstract
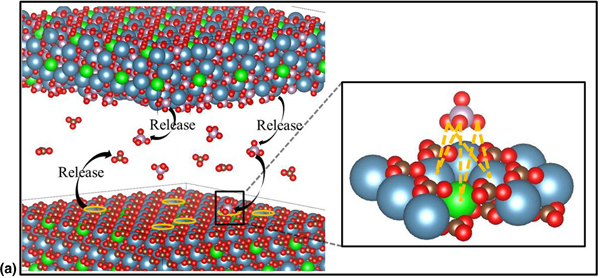
In this study, Mg-substituted tricalcium phosphate (Mg-TCP) nanoparticles were synthesized by hydrothermal reactions of Mg-calcite mesocrystals from echinoderm skeletons. Following the biomineralization of echinoderms, Mg-calcite powder was synthesized via the solid-state transition of Mg-amorphous calcium carbonate prepared by a wet-chemical precipitation method, which can also be used to fabricate Mg-TCP. We illustrated that Mg-calcite with a certain level of Mg substitution led to the formation of Mg-TCP through the ion-exchange reactions in the hydrothermal system. Therefore, this study provides a new pathway for the synthesis of Mg-TCP nanoparticles.
- Type
- Research Letters
- Information
- Copyright
- Copyright © The Author(s) 2019
References
- 3
- Cited by




