Article contents
Grafting of glycerol methacrylate onto silicone rubber using γ-rays: derivatization to 2-oxoethyl methacrylate and immobilization of lysozyme
Published online by Cambridge University Press: 13 February 2018
Abstract
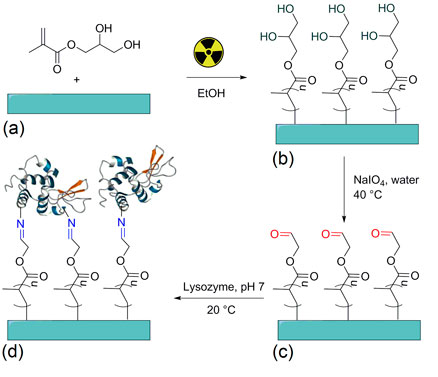
The goal of this work was the modification of silicone rubber (SR) by radiation grafting of glycerol methacrylate (GlyMA) which was limited just on the surface, allowing the control of hydrophilicity and swelling properties. The grafted SRs were activated by derivatization of GlyMA to 2-oxoethyl methacrylate using sodium periodate, enabling the chemical immobilization of lysozyme by covalent bonds. The presence of lysozyme was confirmed by non-specific assay and by the enzymatic activity at 30 °C with Micrococcus lysodeikticus (coccus, Gram-positive). The materials were characterized by Fourier transform infrared spectroscopy-attenuated total reflectance, thermogravimetric analysis, water contact angle, and by mechanical properties as well as scanning electron microscope.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2018
References
- 5
- Cited by




