Article contents
Fluorescence loss of commercial aqueous quantum dots during preparation for bioimaging
Published online by Cambridge University Press: 29 April 2019
Abstract
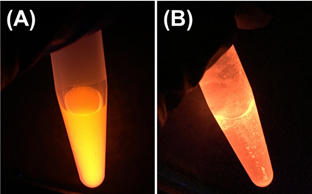
Quantum dots (QDs) are increasingly employed in biologic imaging applications; however, anecdotal reports suggest difficulties in QD bioconjugation. Further, the stability of commercial QDs during bioconjugation has not been systematically evaluated. Thus, we examined fluorescence losses resulting from aggregation and declining photoluminescence quantum yield (QY) for commercial CdSe/ZnS QD products from four different vendors. QDs were most stable in the aqueous media in which they were supplied. The largest QY declines were observed during centrifugal filtration, whereas the largest declines in colloidal stability occurred in 2-(N-morpholino)ethanesulfonic acid (MES) buffer. These results enable optimization of bioconjugation protocols.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2019
Footnotes
These authors have contributed equally to this work.
References
- 4
- Cited by




