Article contents
Bimodal nanoporous platinum on sacrificial nanoporous copper for catalysis of the oxygen-reduction reaction
Published online by Cambridge University Press: 22 November 2018
Abstract
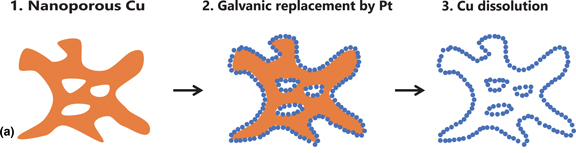
Bimodal nanoporous platinum (BNP-Pt) is synthesized by using a sacrificial nanoporous copper (NP-Cu) support for oxygen-reduction-reaction (ORR) catalysts in fuel cells. The specific ORR catalytic activity of BNP-Pt increases by the dissolution and removal of supporting NP-Cu, suggesting that the BNP structure improves the intrinsic catalytic properties of platinum. The lattice contraction of BNP-Pt containing residual copper even after NP-Cu removal is milder than predicted by Vegard's law. The BNP structure governs the intrinsic catalytic activity of the platinum by relaxing the lattice contraction and by alloying with copper and/or misfit strain at the Pt/Cu interface.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2018
References
- 5
- Cited by




