Article contents
Bicomponent nanofibrous scaffolds with dual release of anticancer drugs and biomacromolecules
Published online by Cambridge University Press: 04 March 2019
Abstract
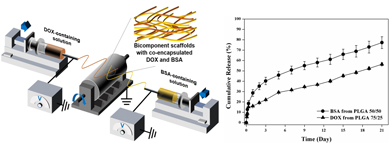
Multifunctional scaffolds with dual release of small molecular drugs and biomacromolecules have potential in many applications such as cancer postoperative care, which require appropriate administration of anticancer drugs and biomacromolecules in a spatiotemporal manner. Herein, a systematic investigation into the dual release of anticancer drugs and biomacromolecules from the bicomponent nanofibrous scaffolds is performed. Their release behavior is affected by different fabrication techniques and different polymers used. We show that the bicomponent scaffold fabricated by dual-source dual-power emulsion electrospinning enables dual release of anticancer drugs and biomacromolecules in a controlled manner, holding promise for combinational cancer postoperative care.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2019
References
- 7
- Cited by




