Article contents
Rapid and scalable synthesis of crystalline tin oxide nanoparticles with superior photovoltaic properties by flame oxidation
Published online by Cambridge University Press: 19 September 2017
Abstract
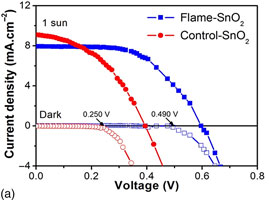
Tin oxide (SnO2) nanoparticles in gram scale quantity were synthesized from inexpensive Sn feedstock by flame oxidation. Selection of optimal feedstock size based on computational fluid dynamics ensures complete conversion of Sn into SnO2 nanoparticles. The rapid melting and oxidation of feedstock in high-temperature oxidative flame endow the crystalline and phase-pure SnO2 nanoparticles, as evident from x-ray diffraction and transmission electron microscopy analysis. Dye-sensitized solar cells fabricated using flame-SnO2 nanoparticles show higher efficiency (ɳ = 2.72%) than that of commercial SnO2 nanoparticles (ɳ = 1.53%). The increased efficiency is attributed to suppression of electron recombination caused by passivation of sub-band-edge surface states.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2017
References
- 5
- Cited by





