No CrossRef data available.
Article contents
Plastic deformation affecting anodic dissolution in electrochemical migration
Published online by Cambridge University Press: 23 May 2019
Abstract
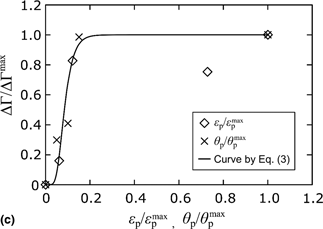
This study investigated the effect of plastic deformation on anodic dissolution in electrochemical migration (ECM) through the growth of deposits. The morphology of deposits synthesized by ECM was analyzed using scanning electron microscopy, where sponge-shaped deposits were observed on the cathode electrode. The mechanism of anodic dissolution was examined by experimentally measuring the variation in the mass of electrodes. The increase and saturation of anodic dissolution in ECM with plastic deformation were observed and were empirically formulated in terms of the change in activation energy. Thus, plastic deformation is proposed as a promising parameter that contributes to controlling ECM.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2019




