Article contents
Electrochemical kinetics and dimensional considerations at the nanoscale: the influence of the density of states
Published online by Cambridge University Press: 14 September 2017
Abstract
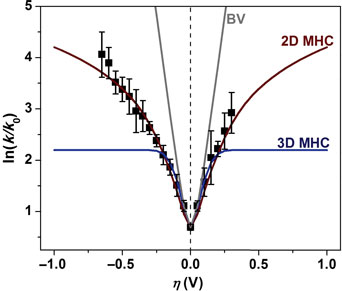
The theories to describe the rate at which electrochemical reactions proceed do not consider explicitly the dimensionality or the occupancy of the energy levels of nanostructured electrodes. It is shown here that the density of states variation in nanoscale electrochemical systems yield novel modulations in the rate constant and concomitant electrical currents. The proposed models extend the utility of presently used Marcus–Hush–Chidsey kinetics to a larger class of materials and could be used as a test of dimensional character. The new models are applied to explain the experimental variation of the electrochemical rate constant of single-layer graphene.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2017
References
- 3
- Cited by




