Article contents
Theoretical and Experimental Studies of Ceramic:Metal Wetting
Published online by Cambridge University Press: 29 November 2013
Extract
Surface phenomena, including wetting, have surely fascinated humans foi millennia. The breakup of a falling stream of water and the behavior of froths, both driven by surface energy, certainly caught the interest of people long before records were kept. The Phoenicians are credited with inventing that remarkable wetting agent, soap, in about 600 B.C., and a 2000-year-old soap factory was unearthed in the ruins of Pompeii.
It is hard to pinpoint the first scientific studies of surfaces and wetting, but it is a good guess that the earliest scientists, or natural philosophers, were interested. In 1805 Young noted that in a system consisting of a liquid and a solid phase, the former will wet the latter to a degree dictated by the surface energies of the system. If a liquid drop rests on a solid substrate, as shown in Figure 1, the wetting angle, θ, characterizes the wetting of solid by liquid. The fundamental relationship between wetting angle and surface energy in equilibrium was given by Young as:
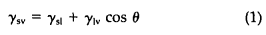
where γsv is the surface energy of solid, γsl is the solid-liquid interfacial energy, γlv is the surface energy of liquid, and θ is the wetting angle.
- Type
- Engineered Interfaces in Composites
- Information
- Copyright
- Copyright © Materials Research Society 1991
References
- 32
- Cited by




