The same excellent optoelectronic properties that make perovskites promising for solar cells also make them ideal for detecting x-rays and gamma rays. The best perovskite-based x-ray detector is more than 1000 times more sensitive than commercial detectors based on amorphous selenium. However, conventional lead halide perovskites lack stability, and the presence of lead is a concern.
Yang Yang of Zhejiang University in China and his colleagues have made a high-performance x-ray detector using single crystals of the perovskite-like material (NH4)3Bi2I9. The device exhibits a very high x-ray sensitivity of 8.2 × 103 µC Gyair–1 cm–2 in a parallel configuration, where electricity flows parallel to its crystal surface. The perpendicular direction device, on the other hand, has a very low detection limit of 55 nGyair/s. This is comparable to previously made cesium bromide-based perovskite x-ray detectors and 100 times lower than the 5.5 µGyair/s required for medical diagnostics.
Single crystals of the material are easy to grow in solution at a low temperature, which makes it a promising nontoxic candidate for fabricating large flat-panel x-ray detectors that are low-cost, green, and potentially flexible, the researchers wrote in Nature Photonics (doi:10.1038/s41566-019-0466-7).
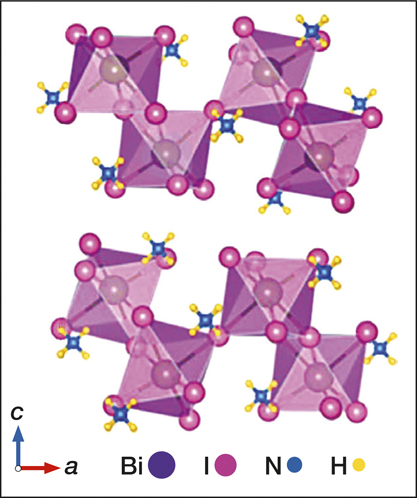
Crystal structure of (NH4)3Bi2l9. Credit: Nature Photonics.
Instability remains the biggest hurdle in the way of perovskites’ practical impact. Adding a small amount of fluoride to perovskites can help, researchers have found.
Ion vacancies are a common defect in organic–inorganic halide perovskite crystals that lead to material degradation. They allow anions and cations to migrate to the surface or grain boundaries and trigger reactions that can cause the organic methylamine or formamidine species to escape. Previously, researchers have added halide and other small ions to suppress these defects, but “most strategies focus on passivating or preventing only one type of defect, either the organic cation or the halide anion vacancy,” Huanping Zhou of Peking University and his colleagues wrote in a Nature Energy (doi:10.1038/s41560-019-0382-6) paper.
The research team decided to use highly reactive fluoride ions. They added a small amount of sodium halide to state-of-the-art cesium formamidinium-methylammonium lead halide perovskites. Solar cells made with the material had a certified power-conversion efficiency of 21.3%. Even without encapsulation, they retained 90% of this efficiency after 1000 hours of simulated sun exposure.
Using density functional theory calculations, the researchers concluded that fluoride ions are effective at suppressing both types of vacancies by forming strong hydrogen bonds with organic cations and strong ionic bonds with lead in the perovskite film.
By using molecular dynamics and density functional theory calculations, researchers have solved the mystery behind a seemingly contradictory property of halide perovskites. Unlike other efficient optoelectronic materials, atoms in perovskites do not oscillate in a well-defined, harmonic way. Such atomic-level disorder does not typically relate to good sunlight absorption.
David A. Egger and Christian Gehrmann from Universität Regensburg analyzed how thermal vibrations in perovskites affect the spatial correlations in the disorder potential for electrons and holes. They found that large nuclear motion in halide perovskites shortened the length at which the disorder occurred. The disorder remained confined to small volumes of the crystal without affecting other crystal domains. The results were published in Nature Communications (doi:10.1038/s41467-019-11087-y).
The light emission lifetime from a family of perovskite-like tin halides depends strongly on temperature, researchers reported in the journal Nature Materials (doi:10.1038/s41563-019-0416-2). They used the materials to make a high-resolution remote thermal imaging device.
Thermal imagers are used in medicine, defense, and security cameras and to inspect buildings and pipelines. Conventional devices detect infrared radiation from objects. But another promising approach is to use temperature-sensitive photoluminescent materials that emit visible light. These materials can be integrated into objects as temperature probes. To measure the object’s temperature, the material is hit with laser pulses, and then the temperature-dependent photoluminescent decay is measured. These emitters need to be thermally sensitive (less than 0.1°C) over a large temperature range.
After testing several tin halide perovskite compounds, ETH Zürich’s Sergii Yakunin and Maksym V. Kovalenko and their colleagues shortlisted three materials with suitable thermographic properties. The materials could reproducibly measure temperature down to 0.013°C over a range of 100°C. Using low-cost hardware for fluorescence lifetime imaging, the research team was able to use one of the perovskites (C4N2H14I)4SnI6 to make a sensitive, real-time thermal recording.




