Article contents
The thermal expansion of aluminate- and aluminogermanate-sodalites
Published online by Cambridge University Press: 05 July 2018
Synopsis
Discontinuities occurring in the expansion behaviour of aluminosilicate-sodalites with large cavity anions such as I− and 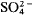 are thought to occur when the coordinate of the cavity cation becomes 0.25 (Henderson and Taylor, 1978). We also suggested that further thermal expansion work could explore how the disposition of sodalites in a diagram such as fig. 4 of Henderson and Taylor relates to the presence or absence of discontinuities.
are thought to occur when the coordinate of the cavity cation becomes 0.25 (Henderson and Taylor, 1978). We also suggested that further thermal expansion work could explore how the disposition of sodalites in a diagram such as fig. 4 of Henderson and Taylor relates to the presence or absence of discontinuities.
We have now studied the expansion of three more sodalites which were expected to show discontinuities, namely one aluminogermanate-sodalite (Na8(Al6Ge6O24)I2) and two aluminate-sodalites (Sr8(Al12O24)(CrO4)2 and Sr2Ba6(Al12 O24)(SO4)2). Sr8(Al12O24)(CrO4)2 was thought to have an ∼ 9 Å cubic cell at room temperature but the occurrence of broadened and split reflections in our sample suggests that this is not the case. Stepwise heating experiments on our sample showed the presence of a reversible, presumably displacive, transformation at 40±2 °C above which the X-ray reflections were sharp.
All three sodalites showed significantly smaller expansion rates than for aluminosilicate-sodalites having similar degrees of structural collapse. The lower mean expansion coefficient (0–500 °C) for Na8(Al6Ge6O24)I2 of 12.5 × 10−6C−1 compared with that for Na8(Al6Si6O24)I2 of 15.1 × 10−6C−1 is particularly significant as the only chemical difference between these two phases is substitution of Ge for Si. In addition none of the three sodalites showed the discontinuities expected and this suggests that the low expansion rates do not allow the cavity cation to reach a coordinate of 0.25 over the temperature range investigated.
Our earlier concept of the mechanism of expansion of the sodalite structure assumed that the expansion of the cavity cation-cavity anion bond forced the cavity cations against and between the framework oxygens so untwisting the partially collapsed structure (Henderson and Taylor, 1978). It now appears that this concept was over-simplified and that the expansion characteristics of sodalites depend on the nature of the tetrahedrally coordinated framework cations present as well as on the cavity cations and anions.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 1979
Footnotes
Present address: 15 Leigh Road, Congleton, Cheshire.
References
Reference
- 4
- Cited by




