Article contents
Synchronous intracrystalline δ13C and δ18O differences in natural calcite crystals
Published online by Cambridge University Press: 05 July 2018
Abstract
New intersectorial isotope data are presented for two calcites. Mendip calcite shows no detectable δ13C and δ18O differences between synchronous {21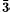 4} and {01
4} and {01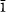 4} sectors. Nottingham calcite shows a δ13C difference of 1.0‰ and a δ18O difference of 0.5‰ between synchronous {40
4} sectors. Nottingham calcite shows a δ13C difference of 1.0‰ and a δ18O difference of 0.5‰ between synchronous {40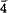 1} and {0001} sectors. Data now available for 5 different calcites show the magnitude of intersectorial fractionation is variable; the effect on δ13C is twice that on δ18O. All five natural calcites also show erratic isotopic differences between symmetrically-equivalent synchronous sectors. Intersectorial isotopic fractionation is probably widespread but its cause and quantification require further work. Synchronous intracrystalline isotopic differences should be accommodated in the interpretation of isotopic data and should influence future sampling strategy.
1} and {0001} sectors. Data now available for 5 different calcites show the magnitude of intersectorial fractionation is variable; the effect on δ13C is twice that on δ18O. All five natural calcites also show erratic isotopic differences between symmetrically-equivalent synchronous sectors. Intersectorial isotopic fractionation is probably widespread but its cause and quantification require further work. Synchronous intracrystalline isotopic differences should be accommodated in the interpretation of isotopic data and should influence future sampling strategy.
- Type
- Mineralogy
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 1997
References
- 8
- Cited by




