Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
KOLITSCH, Uwe
and
PRING, Allan
2001.
Crystal chemistry of the crandallite, beudantite and alunite groups: a review and evaluation of the suitability as storage materials for toxic metals.
Journal of Mineralogical and Petrological Sciences,
Vol. 96,
Issue. 2,
p.
67.
Mills, S. J.
Kampf, A. R.
Raudsepp, M.
and
Christy, A. G.
2009.
The crystal structure of Ga-rich plumbogummite from Tsumeb, Namibia.
Mineralogical Magazine,
Vol. 73,
Issue. 5,
p.
837.
Frost, Ray L.
Bahfenne, Silmarilly
Čejka, Jiří
Sejkora, Jiří
Plášil, Jakub
Palmer, Sara J.
Keeffe, Eloise C.
and
Němec, Ivan
2011.
Dussertite BaFe3+3(AsO4)2(OH)5—a Raman spectroscopic study of a hydroxy‐arsenate mineral.
Journal of Raman Spectroscopy,
Vol. 42,
Issue. 1,
p.
56.
Frost, Ray L.
Xi, Yunfei
and
Pogson, Ross E.
2012.
Raman spectroscopic study of the mineral arsenogorceixite BaAl3AsO3(OH)(AsO4,PO4)(OH,F)6.
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy,
Vol. 91,
Issue. ,
p.
301.
Courtin-Nomade, Alexandra
Rakotoarisoa, Ony
Bril, Hubert
Grybos, Malgorzata
Forestier, Lionel
Foucher, Frédéric
and
Kunz, Martin
2012.
Weathering of Sb-rich mining and smelting residues: Insight in solid speciation and soil bacteria toxicity.
Geochemistry,
Vol. 72,
Issue. ,
p.
29.
Cooper, M. A.
and
Hawthorne, F. C.
2012.
Refinement of the crystal structure of zoned philipsbornite–hidalgoite from the Tsumeb mine, Namibia, and hydrogen bonding in the D2+G3+3(T5+O4)(TO3OH)(OH)6 alunite structures.
Mineralogical Magazine,
Vol. 76,
Issue. 4,
p.
839.
López, Andrés
Xi, Yunfei
and
Frost, Ray L.
2013.
Raman and infrared spectroscopic study of the mineral goyazite SrAl3(PO4)2(OH)5·H2O.
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy,
Vol. 116,
Issue. ,
p.
204.
McCalla, Eric
Abakumov, Artem
Rousse, Gwenaelle
Reynaud, Marine
Sougrati, Moulay Tahar
Budic, Bojan
Mahmoud, Abdelfattah
Dominko, Robert
Van Tendeloo, Gustaaf
Hermann, Raphael P.
and
Tarascon, Jean-Marie
2015.
Novel Complex Stacking of Fully-Ordered Transition Metal Layers in Li4FeSbO6 Materials.
Chemistry of Materials,
Vol. 27,
Issue. 5,
p.
1699.
Pekov, I. V.
Khanin, D. A.
Yapaskurt, V. O.
Pakunova, A. V.
and
Ekimenkova, I. A.
2016.
Minerals of the beudantite–segnitite series from the oxidation zone of the Berezovskoe gold deposit, middle Urals: Chemical variations, behavior of admixtures, and antimonian varieties.
Geology of Ore Deposits,
Vol. 58,
Issue. 7,
p.
600.
Wierzbicka-Wieczorek, Maria
Kolitsch, Uwe
Panczer, Gerard
Giester, Gerald
Chanmuang, Chutimun
and
Grzechnik, Andrzej
2017.
Crystal structures and photoluminescence properties of two novel sorosilicates with an unprecedented ratio of di- and trisilicate groups: Ba 2 Ho 10 (Si 2 O 7 ) 3 (Si 3 O 10 ) 2 and isotypic Ba 2 Er 10 (Si 2 O 7 ) 3 (Si 3 O 10 ) 2.
Journal of Solid State Chemistry,
Vol. 252,
Issue. ,
p.
33.
Annamalai, Alagappan
Sandström, Robin
Gracia-Espino, Eduardo
Boulanger, Nicolas
Boily, Jean-François
Mühlbacher, Inge
Shchukarev, Andrey
and
Wågberg, Thomas
2018.
Influence of Sb5+as a Double Donor on Hematite (Fe3+) Photoanodes for Surface-Enhanced Photoelectrochemical Water Oxidation.
ACS Applied Materials & Interfaces,
Vol. 10,
Issue. 19,
p.
16467.
Hudson-Edwards, Karen A.
2019.
Uptake and release of arsenic and antimony in alunite-jarosite and beudantite group minerals.
American Mineralogist,
Vol. 104,
Issue. 5,
p.
633.
Mereiter, Kurt
Walter, Franz
and
Bojar, Hans-Peter
2023.
Mallestigite, Pb3Sb(SO4)(AsO4)(OH)6·3H2O, from the type locality – new data, crystal structure, and structural relationships.
Mineralogy and Petrology,
Vol. 117,
Issue. 4,
p.
761.
Ondrejka, Martin
Ferenc, Štefan
Majzlan, Juraj
Števko, Martin
Kopáčik, Richard
Voleková, Bronislava
Milovská, Stanislava
Göttlicher, Jörg
Steininger, Ralph
Mikuš, Tomáš
Uher, Pavel
Biroň, Adrián
Sejkora, Jiří
and
Molnárová, Alexandra
2023.
Secondary uranyl arsenates–phosphates and Sb–Bi-rich minerals of the segnitite–philipsbornite series in the oxidation zone at the Prakovce-Zimná Voda REE–U–Au quartz-vein mineralisation, Western Carpathians, Slovakia.
Mineralogical Magazine,
Vol. 87,
Issue. 6,
p.
849.
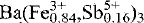 (AsO4)2(OH,H2O)6, has been refined in space group R3̄m with a 7.410(3), c 17.484(4) Å, Z = 3, to R = 3.2 % and Rw = 3.7 % using 377 observed reflections with I ≥ 3 σ(I). The structure is of the alunite-type and consists of sheets of corner-sharing (Fe3+,Sb5+)O6 octahedra parallel to (0001). The substitution of Sb5+ for Fe3+, and not for As5+, is unambiguously demonstrated not only by the structure refinement but also by electron microprobe analyses and crystal-chemical considerations. The icosahedrally coordinated Ba cations occupy cavities between pairs of octahedral sheets and are surrounded by six O atoms from the AsO4 tetrahedra and six O atoms from the (Fe3+,Sb5+)O6 octahedra. The mean bond lengths for the various coordination polyhedra are As-O 1.684(3) Å, (Fe,Sb)-O 2.004(1) Å, and Ba-O 2.872(2) Å. A hydrogen-bonding network is modelled using bond-valence calculations. The dussertite sample investigated is the first member of the crandallite group found to contain substantial Sb.
(AsO4)2(OH,H2O)6, has been refined in space group R3̄m with a 7.410(3), c 17.484(4) Å, Z = 3, to R = 3.2 % and Rw = 3.7 % using 377 observed reflections with I ≥ 3 σ(I). The structure is of the alunite-type and consists of sheets of corner-sharing (Fe3+,Sb5+)O6 octahedra parallel to (0001). The substitution of Sb5+ for Fe3+, and not for As5+, is unambiguously demonstrated not only by the structure refinement but also by electron microprobe analyses and crystal-chemical considerations. The icosahedrally coordinated Ba cations occupy cavities between pairs of octahedral sheets and are surrounded by six O atoms from the AsO4 tetrahedra and six O atoms from the (Fe3+,Sb5+)O6 octahedra. The mean bond lengths for the various coordination polyhedra are As-O 1.684(3) Å, (Fe,Sb)-O 2.004(1) Å, and Ba-O 2.872(2) Å. A hydrogen-bonding network is modelled using bond-valence calculations. The dussertite sample investigated is the first member of the crandallite group found to contain substantial Sb.



