Article contents
Pb-bearing hollandite-type titanates: A first natural occurrence and reconnaissance synthesis study
Published online by Cambridge University Press: 05 July 2018
Abstract
Some samples of hollandite-type titanates from the Murun alkaline complex (Yakutia, Russia) contain appreciable amounts of Pb (up to 12.5 wt.% PbO). These titanates occur in a pegmatitic K-feldsparaegirine rock containing subordinate K-rich batisite, titanite, wadeite and other minerals. The Pb-bearing crystals coexist with hollandite-type phases devoid of detectable Pb and zoned from a Kdominant (priderite) core to a Ba-dominant (henrymeyerite) rim. Recalculation of the microprobe analyses on the stoichiometric basis indicates that most of the Fe occurs in this mineral in trivalent form, suggesting the existence of a solid solution between the Ba(Ti6Fe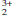 )O16, K2(Ti6Fe
)O16, K2(Ti6Fe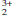 )O16 and Pb(Ti6Fe
)O16 and Pb(Ti6Fe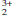 )O16 end-members. The maximum proportion of the latter end-member in the Murun titanates is ∽45 mol.%. The Ba-free compositions [Pb1.0–1.3(Ti,Fe)8O16] and intermediate members of the (Ba1–xPbx)(Ti6Fe
)O16 end-members. The maximum proportion of the latter end-member in the Murun titanates is ∽45 mol.%. The Ba-free compositions [Pb1.0–1.3(Ti,Fe)8O16] and intermediate members of the (Ba1–xPbx)(Ti6Fe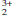 )O16 series were synthesized at 1050 –1100ºC. The synthesis products comprise tetragonal hollandites of various stoichiometry intermixed with rutile, a pseudobrookite-type phase and (for the Ba-free compositions) minor macedonite. Electron microprobe analyses of the hollandites indicate that there is a continuous series of compositions between the two hexatitanate end-members, Ba(Ti6Fe
)O16 series were synthesized at 1050 –1100ºC. The synthesis products comprise tetragonal hollandites of various stoichiometry intermixed with rutile, a pseudobrookite-type phase and (for the Ba-free compositions) minor macedonite. Electron microprobe analyses of the hollandites indicate that there is a continuous series of compositions between the two hexatitanate end-members, Ba(Ti6Fe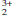 )O16 and Pb(Ti6Fe
)O16 and Pb(Ti6Fe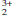 )O16. The crystal structure of one intermediate member was refined by the Rietveld method in space group I4/m, and found to differ from the hollandite archetype (i.e. Pb-bearing Ba manganate) in that Pb is preferentially partitioned into the 2b tunnel site at (0,0,½), whereas Ba is partitioned into the larger 4e site at (0,0,∽0.8).
)O16. The crystal structure of one intermediate member was refined by the Rietveld method in space group I4/m, and found to differ from the hollandite archetype (i.e. Pb-bearing Ba manganate) in that Pb is preferentially partitioned into the 2b tunnel site at (0,0,½), whereas Ba is partitioned into the larger 4e site at (0,0,∽0.8).
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2003
References
- 5
- Cited by




