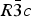Introduction
The mineral mertieite, with general formula Pd5+x(Sb,As)2–x was discovered in the Au-Pt concentrates at Goodnews Bay, Alaska (Desborough et al., Reference Desborough, Finney and Leonard1973). Those authors derived two types of composition for mertieite grains: mertieite-I, (Pd5.04Cu0.4)(Sb0.92As0.9) and mertieite-II, (Pd5.13Cu0.02)(Sb1.53As0.33).
After a few years the mertieite minerals were re-examined (Cabri et al., Reference Cabri, Laflamme, Stewart, Rowland and Chen1975) and it was established that the composition of mertieite-II corresponds to Pd8(Sb,As)3, and the parameters of its rhombohedral unit cell are: a = 7.55 Å, c = 43.18 Å. Later Cabri (Reference Cabri1980) gave the structural formula for the mineral as Pd16Sb5As (Z = 6) with space group ![]() $R\bar 3c$ based on an unpublished crystal-structure determination. Arsenic atoms were located in a special position (As1), and the Sb to As ratio was found to be equal to 5:1.
$R\bar 3c$ based on an unpublished crystal-structure determination. Arsenic atoms were located in a special position (As1), and the Sb to As ratio was found to be equal to 5:1.
McDonald and Cabri (Reference McDonald and Cabri2005) refined the crystal structure of mertieite-II up to wR2 = 7.68%. The structural formula was proposed as Pd48Sb15As3. The important role of single-crystal X-ray diffraction (XRD) in the study of mertieite-group minerals was emphasized.
The crystal structure of the synthetic antimony analogue of mertieite-II, Pd8Sb3, was determined by Wopersnow and Schubert (Reference Wopersnow and Schubert1976) using single-crystal XRD. The structural model proposed was a non-centrosymmetric space group R3c, a = 7.6152, c = 43.032 Å, V = 2161.1 Å3, Z = 12; the reliability factor R of the solution was 14%. Marsh (Reference Marsh1994) refined the structure using experimental data collected by Woperson to centrosymmetric space group ![]() $RR\bar 3c$; refinement gave R = 12.6%.
$RR\bar 3c$; refinement gave R = 12.6%.
The current study is devoted to the crystal-structure analysis of natural mertieite-II, Pd8(Sb,As)3. The structure was refined successfully and the space group, coordinates of atoms in anisotropic form, site populations, interatomic distances and structural formula were defined.
Material for investigation
The mertieite-II crystals for this structural refinement were received from Dr. Kari Kojonen, Geological Survey of Finland. They were collected from the black sands of gold placers from the Kaarreoja River, Inari commune, Finnish Lapland, Finland (68,65°N, 25,63°E). The most probable source of the platinum-group minerals (PGM) is the mafic-ultramafic layered intrusions represented by gabbros and pyroxenites previously mapped as amphibolites that lie upstream of the sample-collection point (Meriläinen, Reference Meriläinen1976; Kojonen, Reference Kojonen2011).
The PGMs from the black sands in the placer deposit are: sperrylite, isoferroplatinum, native platinum, Pt-oxide, braggite, cooperite, vysotskite, isomertieite, mertieite-I, mertieite-II, Os-Ir-Ru alloys, native Os, rustenburgite, palladseite and miessiite (Kojonen et al. Reference Kojonen, Tarkian, Knauf and Törnroos2005, Reference Kojonen, Tarkian, Knauf, Törnroos and Heidrich2006, Reference Kojonen, Tarkian, Knauf, Törnroos and Heidrich2007, Reference Kojonen, McDonald, Stanley and Johanson2011; Kojonen, Reference Kojonen2011). Further alloys of Pt-Cu, Pt-Pd, Au-Pd, Au-Sn, were reported. Undefined PGM phases are PtPdAs, PtPdAsS2, (Pt,Cu,Fe)Te, Pd5As2 and Pd11Sb3As2. Other heavy minerals discovered include thorianite, native Bi, cassiterite, columbite–tantalite, wolframite and monazite.
Methods of study
Platinum-group mineral nuggets from the heavy fraction were mounted in an epoxy resin and a polished mount was prepared for examination by optical and scanning electron microscopy (SEM).
The chemical composition was determined using a Cameca SX 100 five spectrometer WDS instrument. The quantitative electron microprobe analyses (EMPA) were performed with an operating voltage of 15 kV, a beam current of 20 nA and a beam diameter of 1 µm. Pure Pd, Rh, Pt, cobaltite (for As), Sb2Te3 (for Sb), chalcopyrite (for Cu and S) and hematite (for Fe) were used as reference materials. AsLα (LIF diffracting crystal), PdLα (LIF), SbLα (PET), SKα (PET), FeKα (LIF), RhLα (PET), PtLα (LIF), CuKα (LIF) spectral lines were used in the EPMA. The results obtained were used to select two grains of mertieite-II (Figs 1 and 2) for structural investigation (Table 1).
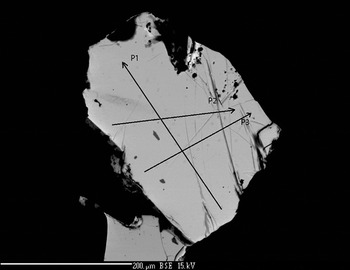
Fig. 1. Mertieite-II crystal I (SEM back-scattered electron image).
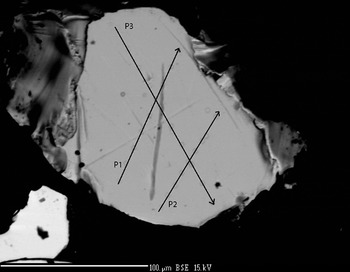
Fig. 2. Mertieite-II crystal II (SEM back-scattered electron image).
Table 1. Representative* compositions of mertieite-II (microprobe).
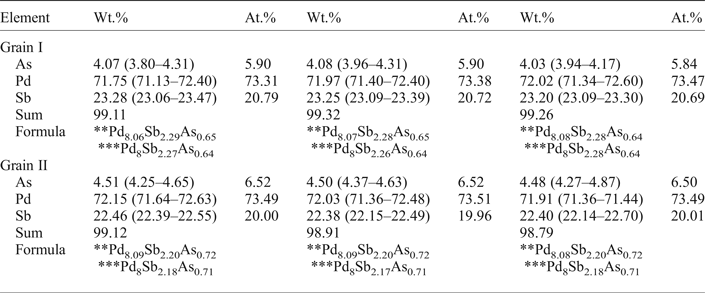
* Average analysis along the profiles.
** Calculated with 11 atoms per formula unit.
*** Calculated with 8 atoms of Pd per formula unit.
The grains were retrieved from the polished mount and analysed by single-crystal X-ray diffraction at the Center of X-ray diffraction studies at St. Petersburg State University (XRD Center SPbSU). Experimental data collection was achieved using an Agilent Technologies Xcalibur Eos diffractometer, which was operated at 50 kV and 40 mA. A hemisphere of three-dimensional data was collected at 293 K using monochromatic MoKα radiation. The data were integrated and corrected by means of the CrysAlisPro (Agilent Technologies, 2012) program package, which was also used to apply empirical absorption correction using spherical harmonics, implemented in the SCALE3 ABSPACK scaling algorithm. The structures were solved by direct methods using the SHELXT program (Sheldrick, Reference Sheldrick2015). Four Pd positions, two Sb positions and one As site were located in the structure. The SHELXL program (Sheldrick, Reference Sheldrick2008) was used for the crystal-structure refinement. The population of the Sb and As sites was refined against Sb/As. The Sb1 and As1 site are occupied completely by Sb and As atoms, respectively. The site-occupancy factors of the M1 site were defined as Sb0.94As0.06 (crystal I) and Sb0.88As0.12 (crystal II). The structures were refined to R1 = 0.0222 for 574 (I) and R1 = 0.0228 for 567 (II) unique observed reflections with |Fo| ≥ 4σF. The crystallographic data and selected refinement parameters are shown in Table 2. The final atomic coordinates and displacement parameters are listed in Tables 3 and 4, and the selected interatomic distances are in Table 5. Table 6 shows the coordination numbers and the average values of the bond lengths.
Table 2. Crystal data and structure refinement for mertieite-II.
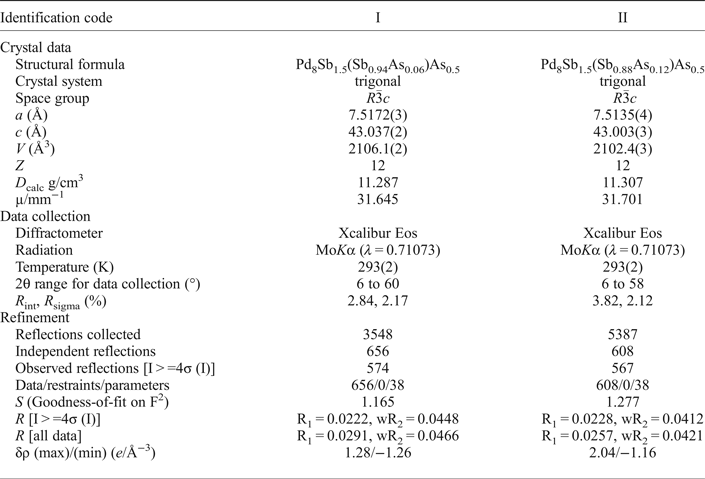
Table 3. Atom coordinates and equivalent isotropic displacement parameters (Å2) for mertieite-II.
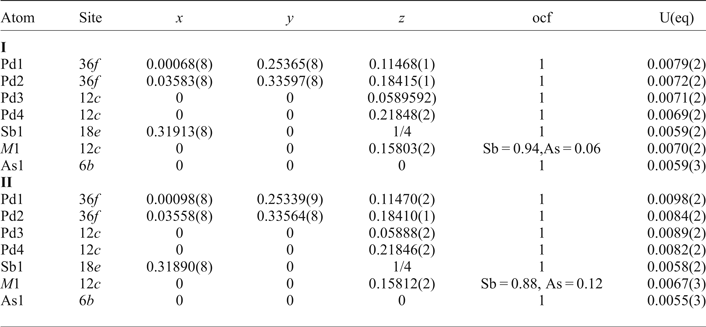
Table 4. Anisotropic displacement parameters (Å2) for mertieite-II.
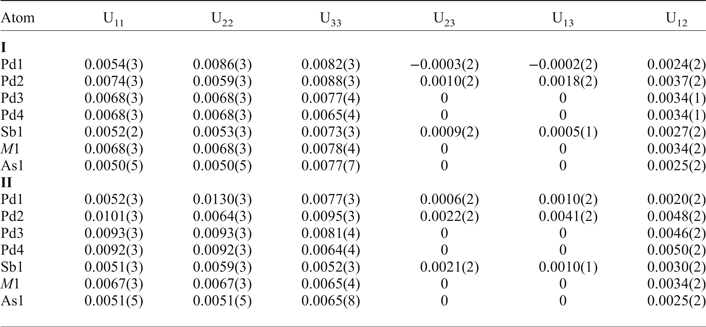
Table 5. Interatomic distances (Å) for mertieite-II (crystal I) and (crystal II).

Table 6. The coordination numbers together with the average values of the Pd–Pd and Pd–Sb/As distances around the different atoms in mertietite-II and Pd8Sb3.
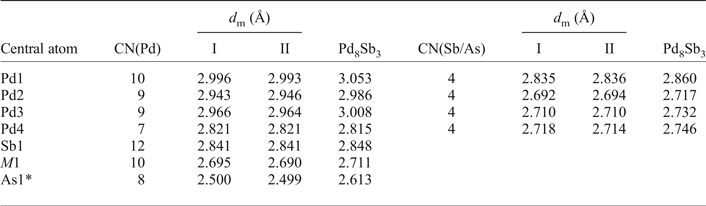
CN(Pd) is the number of nearest Pd neighbours; CN(Sb/As) the number of nearest Sb/As neighbours; d m the average distance in Å.
* Sb3 position on the Pd8Sb3 structure.
Chemical composition
The compositions of the two crystals were determined at ten points along each of three lines across each of the crystals (Figs 1 and 2). Table 1 presents the mean values for each profile. The compositions of the two crystals are nearly identical. In addition to Pd, As and Sb there are minor amounts of S (~0.04–0.05 wt.%) in some analyses. The As content in grain II is slightly higher than in the grain I. The Sb content is a little lower in grain II. The element concentrations vary within very narrow limits (~1 wt. %). The average element contents (in wt.%) are: Pd 71.91, Sb 23.25, As 4.06 (grain I), and Pd 72.03, Sb 22.42, As 4.50 (grain II). They correspond to empirical formulae for crystals I and II, respectively, of: Pd8.07Sb2.29As0.65 (grain I) and Pd8.09Sb2.20As0.72 (grain II) calculated with 11 atoms per unit cell. The mertieite-II formulae calculated on the basis of 8 Pd atoms for samples I and II are Pd8Sb2.27As0.64 and Pd8Sb2.18As0.71, respectively.
The calculated formulae are not completely identical with the general formula of mertieite-II (Table 7). Mertieite-II from the Kaarreoja River is characterized by a greater As content and a lower Sb content compared with mertieite-II from other localities.
Table 7. The compositions of mertieite-II from different localities.

Crystal structure
The structure of mertieite-II contains four symmetrically independent Pd atom positions with two 36f (Pd1 and Pd2) and two 12c (Pd3 and Pd4) Wyckoff positions. The coordination polyhedra are: a Pd1-polyhedron that is a distorted Frank-Kasper polyhedron (14-vertex); the Pd2- and Pd3-polyhedra that are 13-vertex capped distorted icosahedra; and the Pd4 polyhedron that is an 11-vertex pentacapped trigonal prism (Fig. 3).

Fig. 3. Coordination polyhedrons of the mertieite-II structure.
The Sb atoms occupy the positions: 18e (Sb1, Coordination number (CN) = 12, icosahedra) and 12c (M1, CN = 10, a four-capped trigonal prism). Arsenic atoms are located in the 6b position (As1, CN = 8, square prism).
The unit cell of mertieite-II has a very elongated c parameter (~43 Å) (Fig. 4). Two types of triangular 36 nets of Sb and As atoms contribute to the structure (Fig. 5a,b). One type of net is built up only of Sb atoms in the Sb1 position; the other type of net consists of Sb and As atoms in both the M1 and As1 positions. The (M1,As1)-nets are slightly distorted with As and Sb atoms displaced above and below the (110) plane.
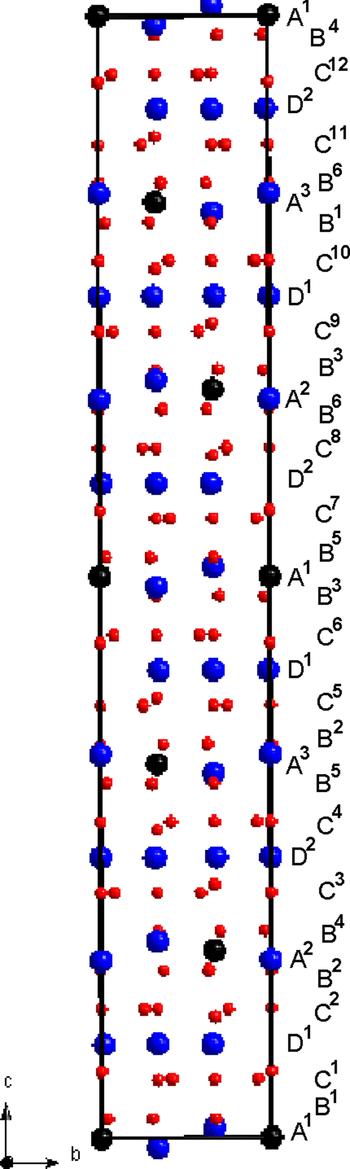
Fig. 4. Projection of the mertieite-II structure along the x axis. Sb atoms are drawn as large blue circles, As atoms as large black circles, Pd atoms as small red circles.

Fig. 5. Nets of atoms in the mertieite-II structure: (a) (Sb,As)-atoms 36 net, (b) Sb-atoms 36 net, (c) Pd-atoms 36 net, (d) Pd-atoms pentagon-triangular 5.33 net. The colour code is the same as in Fig. 4.
Palladium atoms are also located in net-like layers parallel to the (110) plane and there are two types of Pd-net. Palladium atoms in the Pd2 positions form a distorted triangular 36 net (Fig. 5c). Palladium atoms in the Pd1, Pd3 and Pd4 positions form a second Pd-net (Fig. 5d) which consists of pentagons and triangles which is known as a 5.33 net.
The Sb1- and (M1,As1)-nets alternate along the z axis of the unit cell. Nets are also shifted relative to each other in the plane (110). The nets stack in such a way, that the unit cell of mertieite-II contains a total of 36 layers: six Sb1-nets, six (M1,As1)-nets, 12 Pd1-nets and 12 (Pd1,Pd3,Pd4)-nets (Fig. 4). The stacking sequence is: A1B1C1D1C2B2A2B4C3D4C4B5A3B2C5D1C6B3A1B5C7D2C8B6A2B3C9D1C10B1A3B6C11D2C12B4, where the upper indexes denote layers in different orientations (Fig. 4).
The Sb-net and the (Sb,As)-net form a two-dimensional ‘sandwich’-like package (Fig. 6). Palladium atoms occupy all of the octahedral and 5/6 of the tetrahedral interstices of the package. Thus there are two types of Pd-net inside the As,Sb-package the heights of which are ~1/3 of the interlayer distance above and below each As, Sb net (Fig. 6).

Fig. 6. A fragment of the mertieite-II structure: a two-dimensional 4-layer package is shown in the projection viewed down the z axis. The colour code is the same as in Fig. 4. Pd1 atoms (small red circles) occupy the octahedral voids (one octahedral void is shaded). Pd2, Pd3, Pd4 atoms (large red circles) occupy the tetrahedral voids (five tetrahedral voids are shaded).
Discussion
Mertieite-II is isostructural with the Pd8Sb3 structure. The As → Sb substitution of up to 4.50 wt.% in the mertieite-II was found not to affect the main structural topology. The unit-cell dimensions of synthetic Pd8Sb3 are slightly higher (a = 7.6152, c = 43.032 Å, V = 2161.15 Å3) than those of mertieite. The interatomic Pd–Pd distances do not differ significantly between these two structures (Table 6). The main difference between these two structures is the Pd–As and Pd–Sb atomic distances. The As1–Pd distances in the polyhedra around atoms at the 6b site are equal to 2.4878, 2.5322 Å (crystal I) and 2.4874, 2.5371 Å (crystal II) in the mertieite-II structure, in contrast to Sb3–Pd distances of 2.5944 and 2.668 Å, in the Pd8Sb3 structure (Table 6). The volume of the polyhedra around the Sb2 atom site (12e position) also differ significantly. The Sb2–Pd bond lengths are larger in the mertieite-II structure (2.5948 to 2.7896 Å in crystal I and 2.602–2.7916 Å in crystal II) compared with 2.6594 to 2.7685 Å in the Pd8Sb3 structure. The Sb1 polyhedron is highly distorted in natural mertieite-II: the Sb–Pd distances vary from 2.6158 to 3.3407 Å (crystal I), 2.6160–3.3402 Å (crystal II) whereas the Sb1 polyhedron is a little more regular in the Pd8Sb3 structure and the range of the same distances is: 2.6561–3.3029 Å.
Pearson (Reference Pearson1972) proposed a classification of metallic phases based on nets of atoms. The triangular As, Sb and Pd nets of the mertieite-II structure are the close-packed 36 layer according to Pearson's classification. The crystal structures of synthetic Pd8Sb3 and natural mertieite-II, Pd8Sb2.5As0.5, can be derived from the hexagonal close packing by filling additional layers between the close-packed 36 layers.
Crystal-structure analysis of mertieite-II from the Kaarreoja River placer confirmed that As atoms occupy a special position in the structure as indicated by previous studies of the mineral from other localities (Cabri, Reference Cabri1980; McDonald and Cabri, Reference McDonald and Cabri2005). In the present investigation slightly more As-rich crystals were studied. The Sb- and As-site occupancies of the structure were analysed. The formulae of mertieite-II from the Kaarreoja River defined by structural analysis are Pd8Sb1.5(Sb0.94As0.06)As0.5 (crystal I) and Pd8Sb1.5(Sb0.88As0.12)As0.5 (crystal II). These results are in relatively good agreement with formulae calculated from microprobe data: Pd8Sb2.27As0.64 (crystal I) and Pd8Sb2.18As0.71 (crystal II). Mertieite-II crystals from others localities have insufficient As content to completely fill the As1 site (Table 7 and Fig. 7). The Sb content of some of the mertieite-II grains is greater than the capacity of all of the Sb sites in the structure. Therefore, the excess Sb may also occupy the As1 site. The general formula of mertieite-II is Pd8(Sb,As)3 (Cabri, Reference Cabri and Cabri2002). Note, however, that Pd8As3 and Pd8Sb3 are not isostructural. Furthermore, there is no complete solid solution series Pd8As3–Pd8Sb3. The mineral Pd8As2.5Sb0.5 is arsenopalladinite with triclinic symmetry, space group ![]() ${\rm P} \bar 1$ (Karimova et al. unpublished work). The mineral Pd8As3 is stillwaterite. Its structure is still unknown. Only the trigonal system, unit-cell parameters (a = 7.399, c = 10.13 Å) and possible space group P3̄ were determined (Cabri et al., Reference Cabri, Laflamme, Stewart, Rowland and Chen1975).
${\rm P} \bar 1$ (Karimova et al. unpublished work). The mineral Pd8As3 is stillwaterite. Its structure is still unknown. Only the trigonal system, unit-cell parameters (a = 7.399, c = 10.13 Å) and possible space group P3̄ were determined (Cabri et al., Reference Cabri, Laflamme, Stewart, Rowland and Chen1975).
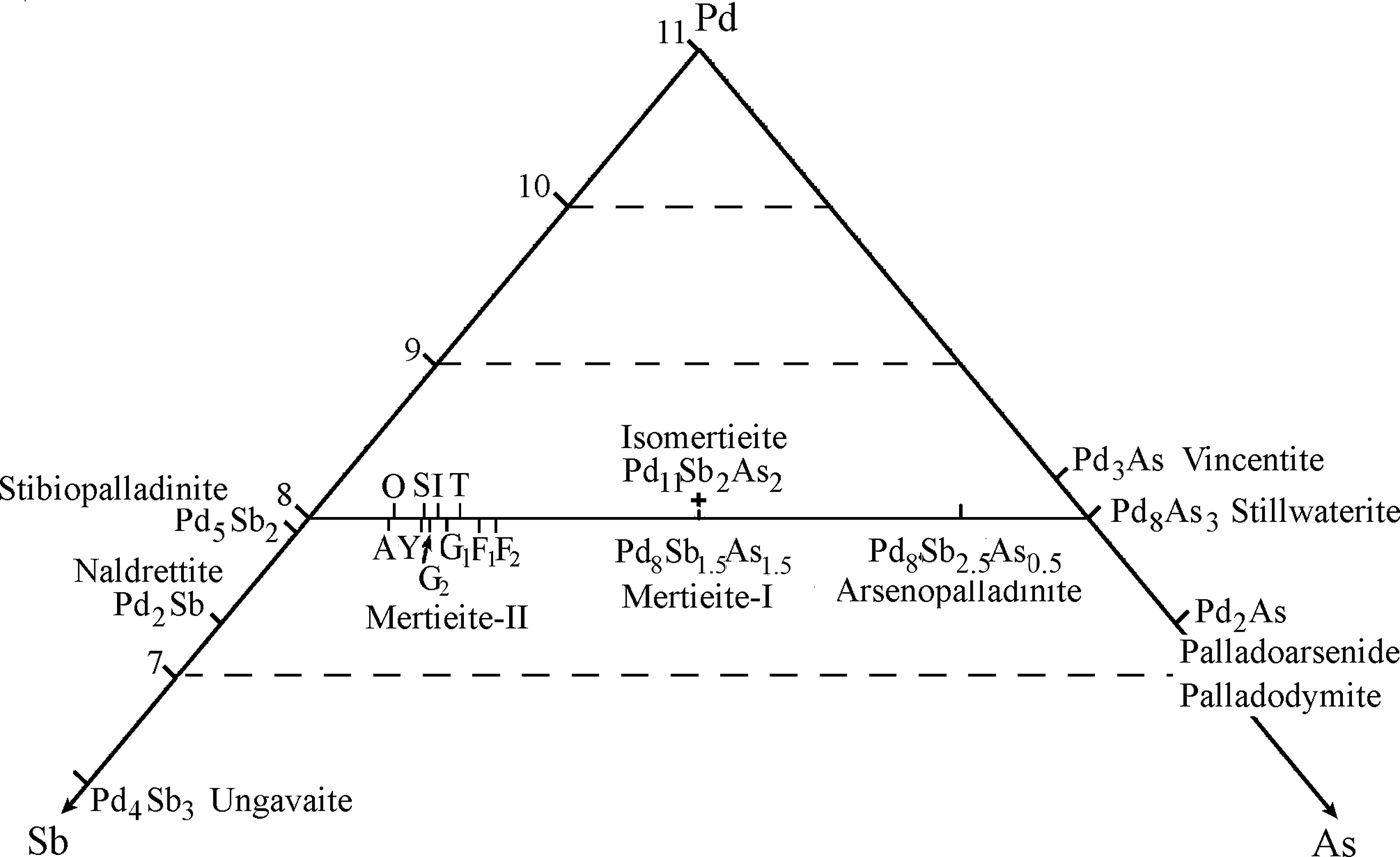
Fig. 7. Part of the Pd-Sb-As diagram: the range of mertieite-II compositions relate to other Pd-Sb-As minerals shown. Mertieite-II – the letters are the place names from Table 7: A = Africa, O = October, Y = Yukon, I = ideal formula, T = Two Duck Lake, S = Sudbury, G1 = Goodnews Bay (Desborough et al., Reference Desborough, Finney and Leonard1973), G2 = Goodnews Bay (Cabri et al., Reference Cabri, Laflamme, Stewart, Rowland and Chen1975), F1 = Finland crystal 1, F2 = Finland crystal 2. Drawn to 11 atoms per formula unit. The small amounts of Sn, Bi, Te, Pb, S has been added to As.
The formula of the asymmetric unit of the mertieite-II structure is Pd2.67Sb0.83As0.17 (Z = 36). It can be presented as Pd16Sb5As1 (Z = 6), where all coefficients are integers.
Pd8Sb2.5As0.5 (Z = 12) is a more convenient form of the formula because the common formula of the mertieite mineral group can be represented as: Pd8T11.5T21T30.5, where T1, T2 and T3 are three possible positions of the Sb and As atoms in the triangular nets of the structure. Different distributions of the Sb and As atoms in these positions are possible. The T3 position is completely occupied by As atoms in mertieite-II, Pd8Sb2.5As0.5, and by Sb atoms in the arsenopalladinite, Pd8As2.5Sb0.5 (Karimova et al., unpublished work). Arsenic atoms should occupy all three positions: T1, T2 and T3, in stillwaterite, Pd8As3. Mertieite-I, is probably a further member of the group. Its formula can be calculated on the basis of 11 atoms: Pd8As1.5Sb1.5. More structural studies of stillwaterite and mertieite-I are required to check the last two hypotheses.
Acknowledgements
The authors acknowledge the generosity of Dr. Kari Kojonen for providing the samples and assistance with the selection and discussion of materials. They are grateful for an insightful review by Dr. František Laufek and the guest editors Drs John Bowles, Nigel Cook, Hannah Hughes, Krister Sundblad and Erik Jonsson for their comments and corrections. They thank Dr. N. Trubkin (IGEM RAS) for SEM/EDS analyses and Prof. Sergey V. Krivovichev and Dr. Vlad V. Gurzhiy for assistance in this scientific research. They also acknowledge the Center of X-ray diffraction studies at St. Petersburg State University (XRD Center SPbSU) for allowing them to collect the experimental data. This study was supported by the Russian Academy of Sciences, Program of Fundamental Research A 72-2 and 72-3.