Article contents
Kihlmanite-(Ce), Ce2TiO2[SiO4](HCO3)2(H2O), a new rare-earth mineral from the pegmatites of the Khibiny alkaline massif, Kola Peninsula, Russia
Published online by Cambridge University Press: 05 July 2018
Abstract
Kihlmanite-(Ce), Ce2TiO2[SiO4](HCO3)2(H2O), is a new rare-earth titanosilicate carbonate, closely related to tundrite-(Ce). It is triclinic, P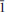 , a = 4.994(2), b = 7.54(2), c = 15.48(4) Å, α = 103.5(4), β = 90.7(2), γ = 109.2(2)o , V = 533(1) Å3, Z = 2 (from powder diffraction data) or a = 5.009(5), b = 7.533(5), c = 15.407(5) Å, α = 103.061(5), β = 91.006(5), γ = 109.285(5)°, V = 531.8(7) Å3, Z = 2 (from single-crystal X-ray diffraction data). The mineral was found in the arfvedsonite-aegirine-microcline vein in fenitized metavolcanic rock at the foot of the Mt Kihlman (Chil’man), near the western contact of the Devonian Khibiny alkaline massif and the Proterozoic Imandra-Varzuga greenstone belt. It forms brown spherulites (up to 2 cm diameter) and sheaf-like aggregates of prismatic crystals, flattened on {010} and up to 0.5 mm diameter. Both spherulites and aggregates occur in interstices in arfvedsonite and microcline, in intimate association with golden-green tundrite-(Ce). Kihlmanite-(Ce) is brown, with a vitreous lustre and a pale yellowish-brown streak. The cleavage is perfect on {010}, parting is perpendicular to c and the fracture is stepped. Mohs hardness is ∼3. In transmitted light, the mineral is yellowish brown; pleochroism and dispersion were not observed. Kihlmanite-(Ce) is biaxial (+), α = 1.708(5), β = 1.76(1), γ = 1.82(1) (589 nm), 2Vcalc = 89°. The optical orientation is Y ^ c = 5°, other details are unclear. The calculated and measured densities are 3.694 and 3.66(2) g cm−3, respectively. The mean chemical composition, determined by electron microprobe, is: Na2O 0.13, Al2O3 0.24, SiO2 9.91, CaO 1.50, TiO2 11.04, MnO 0.26, Fe2O3 0.05, Nb2O5 2.79, La2O3 12.95, Ce2O3 27.33, Pr2O3 2.45, Nd2O3 8.12, Sm2O3 1.67, Gd2O3 0.49 wt.%, with CO2 15.0 and H2O 6.0 wt.% (determined by wet chemical and Penfield methods, respectively), giving a total of 99.93 wt.%. The empirical formula calculated on the basis of Si + Al = 1 atom per formula unit is (Ca0.16Na0.11Mn0.02)∑0.29[(Ce0.98La0.47Pr0.09Nd0.29Sm0.06Gd0.02)∑1.91(Ti0.82Nb0.12)∑0.94O2 (Si0.97Al0.03)∑1O4.02(HCO3)2.01](H2O)0.96. The simplified formula is Ce2TiO2(SiO4)(HCO3)2·H2O. The mineral reacts slowly in cold 10% HCl with weak effervescence and fragmentation into separate plates. The strongest X-ray powder-diffraction lines [listed as d in Å(I) (hkl)] are as follows: 15.11(100)(00
, a = 4.994(2), b = 7.54(2), c = 15.48(4) Å, α = 103.5(4), β = 90.7(2), γ = 109.2(2)o , V = 533(1) Å3, Z = 2 (from powder diffraction data) or a = 5.009(5), b = 7.533(5), c = 15.407(5) Å, α = 103.061(5), β = 91.006(5), γ = 109.285(5)°, V = 531.8(7) Å3, Z = 2 (from single-crystal X-ray diffraction data). The mineral was found in the arfvedsonite-aegirine-microcline vein in fenitized metavolcanic rock at the foot of the Mt Kihlman (Chil’man), near the western contact of the Devonian Khibiny alkaline massif and the Proterozoic Imandra-Varzuga greenstone belt. It forms brown spherulites (up to 2 cm diameter) and sheaf-like aggregates of prismatic crystals, flattened on {010} and up to 0.5 mm diameter. Both spherulites and aggregates occur in interstices in arfvedsonite and microcline, in intimate association with golden-green tundrite-(Ce). Kihlmanite-(Ce) is brown, with a vitreous lustre and a pale yellowish-brown streak. The cleavage is perfect on {010}, parting is perpendicular to c and the fracture is stepped. Mohs hardness is ∼3. In transmitted light, the mineral is yellowish brown; pleochroism and dispersion were not observed. Kihlmanite-(Ce) is biaxial (+), α = 1.708(5), β = 1.76(1), γ = 1.82(1) (589 nm), 2Vcalc = 89°. The optical orientation is Y ^ c = 5°, other details are unclear. The calculated and measured densities are 3.694 and 3.66(2) g cm−3, respectively. The mean chemical composition, determined by electron microprobe, is: Na2O 0.13, Al2O3 0.24, SiO2 9.91, CaO 1.50, TiO2 11.04, MnO 0.26, Fe2O3 0.05, Nb2O5 2.79, La2O3 12.95, Ce2O3 27.33, Pr2O3 2.45, Nd2O3 8.12, Sm2O3 1.67, Gd2O3 0.49 wt.%, with CO2 15.0 and H2O 6.0 wt.% (determined by wet chemical and Penfield methods, respectively), giving a total of 99.93 wt.%. The empirical formula calculated on the basis of Si + Al = 1 atom per formula unit is (Ca0.16Na0.11Mn0.02)∑0.29[(Ce0.98La0.47Pr0.09Nd0.29Sm0.06Gd0.02)∑1.91(Ti0.82Nb0.12)∑0.94O2 (Si0.97Al0.03)∑1O4.02(HCO3)2.01](H2O)0.96. The simplified formula is Ce2TiO2(SiO4)(HCO3)2·H2O. The mineral reacts slowly in cold 10% HCl with weak effervescence and fragmentation into separate plates. The strongest X-ray powder-diffraction lines [listed as d in Å(I) (hkl)] are as follows: 15.11(100)(00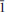 ), 7.508(20)(00
), 7.508(20)(00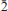 ), 6.912(12)(0
), 6.912(12)(0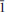 1), 4.993(14)(00
1), 4.993(14)(00 ), 3.563(15)(0
), 3.563(15)(0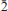 1), 2.896(15)(1
1), 2.896(15)(1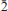
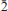 ). The crystal structure of kihlmanite-(Ce) was refined to R1 = 0.069 on the basis of 2441 unique observed reflections (MoKα, 293 K). It is closely related to the crystal structure of tundrite-(Ce) and is based upon [Ce2TiO2(SiO4)(HCO3)2] layers parallel to (001). Kihlmanite-(Ce) can be considered as a cationdeficient analogue of tundrite-(Ce). The mineral is named in honour of Alfred Oswald Kihlman (1858–1938), a remarkable Finnish geographer and botanist who participated in the Wilhelm Ramsay expeditions to the Khibiny Mountains in 1891–1892. The mineral name also reflects its occurrence at the Kihlman (Chil’man) Mountain.
). The crystal structure of kihlmanite-(Ce) was refined to R1 = 0.069 on the basis of 2441 unique observed reflections (MoKα, 293 K). It is closely related to the crystal structure of tundrite-(Ce) and is based upon [Ce2TiO2(SiO4)(HCO3)2] layers parallel to (001). Kihlmanite-(Ce) can be considered as a cationdeficient analogue of tundrite-(Ce). The mineral is named in honour of Alfred Oswald Kihlman (1858–1938), a remarkable Finnish geographer and botanist who participated in the Wilhelm Ramsay expeditions to the Khibiny Mountains in 1891–1892. The mineral name also reflects its occurrence at the Kihlman (Chil’man) Mountain.
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2014
References
- 3
- Cited by




