Article contents
Joteite, Ca2CuAl[AsO4][AsO3(OH)]2(OH)2·5H2O, a new arsenate with a sheet structure and unconnected acid arsenate groups
Published online by Cambridge University Press: 05 July 2018
Abstract
Joteite (IMA2012-091), Ca2CuAl[AsO4][AsO3(OH)]2(OH)2·5H2O, is a new mineral from the Jote mine, Tierra Amarilla, Copiapó Province, Atacama, Chile. The mineral is a late-stage, low-temperature, secondary mineral occurring with conichalcite, mansfieldite, pharmacoalumite, pharmacosiderite and scorodite in narrow seams and vughs in the oxidized upper portion of a hydrothermal sulfide vein hosted by volcanoclastic rocks. Crystals occur as sky-blue to greenish-blue thin blades, flattened and twinned on {001}, up to ~300 μm in length, and exhibiting the forms {001}, {010}, {1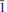 0}, {2
0}, {2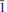 0} and {111}. The blades are commonly intergrown in wheat-sheaf-like bundles, less commonly in sprays, and sometimes aggregated as dense crusts and cavity linings. The mineral is transparent and has a very pale blue streak and vitreous lustre. The Mohs hardness is estimated at 2 to 3, the tenacity is brittle, and the fracture is curved. It has one perfect cleavage on {001}. The calculated density based on the empirical formula is 3.056 g/cm3. It is optically biaxial (–) with α = 1.634(1), β = 1.644(1), γ = 1.651(1) (white light), 2Vmeas = 78(2)° and 2Vcalc = 79.4°. The mineral exhibits weak dispersion, r < v. The optical orientation is X ≈ c*; Y ≈ b*. The pleochroism is Z (greenish blue) > Y (pale greenish blue) > X (colourless). The normalized electron-microprobe analyses (average of 5) provided: CaO 15.70, CuO 11.22, Al2O38.32, As2O546.62, H2O 18.14 (structure), total 100 wt.%. The empirical formula (based on 19 O a.p.f.u.) is: Ca1.98Cu1.00Al1.15As2.87H14.24O19. The mineral is slowly soluble in cold, concentrated HCl. Joteite is triclinic, P
0} and {111}. The blades are commonly intergrown in wheat-sheaf-like bundles, less commonly in sprays, and sometimes aggregated as dense crusts and cavity linings. The mineral is transparent and has a very pale blue streak and vitreous lustre. The Mohs hardness is estimated at 2 to 3, the tenacity is brittle, and the fracture is curved. It has one perfect cleavage on {001}. The calculated density based on the empirical formula is 3.056 g/cm3. It is optically biaxial (–) with α = 1.634(1), β = 1.644(1), γ = 1.651(1) (white light), 2Vmeas = 78(2)° and 2Vcalc = 79.4°. The mineral exhibits weak dispersion, r < v. The optical orientation is X ≈ c*; Y ≈ b*. The pleochroism is Z (greenish blue) > Y (pale greenish blue) > X (colourless). The normalized electron-microprobe analyses (average of 5) provided: CaO 15.70, CuO 11.22, Al2O38.32, As2O546.62, H2O 18.14 (structure), total 100 wt.%. The empirical formula (based on 19 O a.p.f.u.) is: Ca1.98Cu1.00Al1.15As2.87H14.24O19. The mineral is slowly soluble in cold, concentrated HCl. Joteite is triclinic, P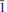 , with the cell parameters: a = 6.0530(2), b = 10.2329(3), c = 12.9112(4) Å, α = 87.572(2), β = 78.480(2), γ = 78.697(2)°, V = 768.40(4) Å3 and Z = 2. The eight strongest lines in the X-ray powder diffraction pattern are [dobs Å (I)(hkl)]: 12.76(100)(001), 5.009(23)(020), 4.206(26)(120,003,121), 3.92(24)(022,0
, with the cell parameters: a = 6.0530(2), b = 10.2329(3), c = 12.9112(4) Å, α = 87.572(2), β = 78.480(2), γ = 78.697(2)°, V = 768.40(4) Å3 and Z = 2. The eight strongest lines in the X-ray powder diffraction pattern are [dobs Å (I)(hkl)]: 12.76(100)(001), 5.009(23)(020), 4.206(26)(120,003,121), 3.92(24)(022,0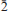 2,
2,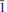 02), 3.40(25)(
02), 3.40(25)(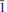 13), 3.233(19)(031,023,123,0
13), 3.233(19)(031,023,123,0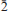 3), 2.97(132,201) and 2.91(15)(
3), 2.97(132,201) and 2.91(15)(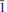 22,
22,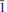 13). In the structure of joteite (R1 = 7.72% for 6003 Fo > 4σF), AsO4 and AsO3 (OH) tetrahedra, AlO6 octahedra and Cu2+O5 square pyramids share corners to form sheets parallel to {001}. In addition, 7- and 8-coordinate Ca polyhedra link to the periphery of the sheets yielding thick slabs. Between the slabs are unconnected AsO3(OH) tetrahedra, which link the slabs only via hydrogen bonding. The Raman spectrum shows features consistent with OH and/or H2O in multiple structural environments. The region between the slabs may host excess Al in place of some As.
13). In the structure of joteite (R1 = 7.72% for 6003 Fo > 4σF), AsO4 and AsO3 (OH) tetrahedra, AlO6 octahedra and Cu2+O5 square pyramids share corners to form sheets parallel to {001}. In addition, 7- and 8-coordinate Ca polyhedra link to the periphery of the sheets yielding thick slabs. Between the slabs are unconnected AsO3(OH) tetrahedra, which link the slabs only via hydrogen bonding. The Raman spectrum shows features consistent with OH and/or H2O in multiple structural environments. The region between the slabs may host excess Al in place of some As.
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2013
References
- 7
- Cited by




