Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Merlino, Stefano
1988.
Gyrolite: Its Crystal Structure and Crystal Chemistry.
Mineralogical Magazine,
Vol. 52,
Issue. 366,
p.
377.
Barnes, Mary W.
and
Scheetz, Barry E.
1989.
The Chemistry of Al-Tobermorite and its Coexisting Phases AT 175°C.
MRS Proceedings,
Vol. 179,
Issue. ,
Miyake, Michihiro
Iwaya, Masaki
Suzuki, Takashi
Kakehi, Hiroyuki
and
Mitsuda, Takeshi
1990.
Aluminum‐Substituted Gyrolite as Cation Exchanger.
Journal of the American Ceramic Society,
Vol. 73,
Issue. 11,
p.
3524.
Macleod, G.
Hall, A. J.
and
Fallick, A. E.
1990.
An applied mineralogical investigation of concrete degradation in a major concrete road bridge.
Mineralogical Magazine,
Vol. 54,
Issue. 377,
p.
637.
Macintyre, J. E.
Daniel, F. M.
and
Stirling, V. M.
1992.
Dictionary of Inorganic Compounds.
p.
1.
Bruton, Carol J.
Phillips, Brian L.
Meike, Annemarie
Martin, Sue
and
Viani, Brian E.
1993.
Cement Minerals at Elevated Temperature: Thermodynamic and Structural Characteristics.
MRS Proceedings,
Vol. 333,
Issue. ,
Suzuki, S.
and
Sinn, E.
1993.
1.4 nm tobermorite-like calcium silicate hydrate prepared at room temperature from Si(OH)4 and CaCl2 solutions.
Journal of Materials Science Letters,
Vol. 12,
Issue. 8,
p.
542.
Bargar, Keith E.
Keith, Terry E.C.
and
Trusdell, Frank A.
1995.
Fluid-inclusion evidence for past temperature fluctuations in the Kilauea East Rift Zone geothermal area, Hawaii.
Geothermics,
Vol. 24,
Issue. 5-6,
p.
639.
Ferraris, Giovanni
Pavese, Alessandro
and
Soboleva, Svetlana V.
1995.
Tungusite: new data, relationship with gyrolite and structural model.
Mineralogical Magazine,
Vol. 59,
Issue. 396,
p.
535.
Ferraris, Giovanni
1997.
Modular Aspects of Minerals.
p.
275.
Zvyagin, Boris B.
1997.
Modular Aspects of Minerals.
p.
345.
Elton, N. J.
Hooper, J. J.
and
Holyer, V. A. D.
1998.
Al-free gyrolite from the Lizard, Cornwall, England.
Mineralogical Magazine,
Vol. 62,
Issue. 2,
p.
271.
Gong, W.L
Wang, L.M
Ewing, R.C
Vernaz, E
Bates, J.K
and
Ebert, W.L
1998.
Analytical electron microscopy study of surface layers formed on the French SON68 nuclear waste glass during vapor hydration at 200°C.
Journal of Nuclear Materials,
Vol. 254,
Issue. 2-3,
p.
249.
Chiang, Ray-Kuang
Kwag, Gwanghoon.
and
Kenney, Malcolm E.
1998.
Dry Scrubbing Technologies for Flue Gas Desulfurization.
p.
115.
Kulik, Dmitrii A.
and
Kersten, Michael
2001.
Aqueous Solubility Diagrams for Cementitious Waste Stabilization Systems: II, End‐Member Stoichiometries of Ideal Calcium Silicate Hydrate Solid Solutions.
Journal of the American Ceramic Society,
Vol. 84,
Issue. 12,
p.
3017.
Winters, Michael A.
Richter, Jesse D.
Sagar, Sangeetha L.
Lee, Ann L.
and
Lander, Russel J.
2003.
Plasmid DNA Purification by Selective Calcium Silicate Adsorption of Closely Related Impurities.
Biotechnology Progress,
Vol. 19,
Issue. 2,
p.
440.
Black, Leon
Garbev, Krassimir
Stemmermann, Peter
Hallam, Keith R.
and
Allen, Geoffrey C.
2003.
Characterisation of crystalline C-S-H phases by X-ray photoelectron spectroscopy.
Cement and Concrete Research,
Vol. 33,
Issue. 6,
p.
899.
Siauciunas, R.
and
Baltakys, K.
2004.
Formation of gyrolite during hydrothermal synthesis in the mixtures of CaO and amorphous SiO2 or quartz.
Cement and Concrete Research,
Vol. 34,
Issue. 11,
p.
2029.
Wang, Qun
Zhang, John P.
Smith, Timothy R.
Hurst, William E.
and
Sulpizio, Thomas
2005.
An electrokinetic study on a synthetic adsorbent of crystalline calcium silicate hydrate and its mechanism of endotoxin removal.
Colloids and Surfaces B: Biointerfaces,
Vol. 44,
Issue. 2-3,
p.
110.
Meller, Nicola
Hall, Christopher
and
Phipps, Jonathan S.
2005.
A new phase diagram for the CaOAl2O3SiO2H2O hydroceramic system at 200°C.
Materials Research Bulletin,
Vol. 40,
Issue. 5,
p.
715.
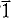 and cell parameters a = 9.74(1), b = 9.74(1), c = 22.40(2)Å, α = 95.7(1)°, β = 91.5(1)°, γ = 120.0(1)°. The structure is built up by the stacking of the structural units already found in the crystal structure of reyerite (Merlino, 1972, 1988), namely tetrahedral sheets S1 and S2 and octahedral sheets O. The tetrahedral and octahedral sheets are connected by corner sharing to give rise to the complex layer which can be schematically described as
and cell parameters a = 9.74(1), b = 9.74(1), c = 22.40(2)Å, α = 95.7(1)°, β = 91.5(1)°, γ = 120.0(1)°. The structure is built up by the stacking of the structural units already found in the crystal structure of reyerite (Merlino, 1972, 1988), namely tetrahedral sheets S1 and S2 and octahedral sheets O. The tetrahedral and octahedral sheets are connected by corner sharing to give rise to the complex layer which can be schematically described as 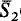
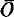 1OS2, where S2 and
1OS2, where S2 and 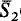 , as well as O and
, as well as O and 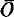 , are symmetry-related units. Successive complex layers with composition [Ca14Si23AlO60(OH)8]-5 are connected through an interlayer sheet made up by calcium and sodium cations and water molecules.
, are symmetry-related units. Successive complex layers with composition [Ca14Si23AlO60(OH)8]-5 are connected through an interlayer sheet made up by calcium and sodium cations and water molecules.



