Article contents
From structure topology to chemical composition. X. Titanium silicates: the crystal structure and crystal chemistry of nechelyustovite, a group III Ti-disilicate mineral
Published online by Cambridge University Press: 05 July 2018
Abstract
The crystal structure of nechelyustovite, ideally Na4Ba2Mn1.5☐2.5Ti5Nb(Si2O7)4O4(OH)3F(H2O)6, a 5.447(1) Å, b 7.157(1) Å, c 47.259(9) Å, α 95.759(4)°, β 92.136(4)°, γ 89.978(4)°, V 1831.7(4) Å3, space group P , Z = 2, Dcalc. 3.041 g cm–3, from Lovozero alkaline massif, Kola Peninsula, Russia, has been solved and refined to R1 = 13.9% on the basis of 1745 unique reflections (Fo > 15σF). Electron microprobe analysis yielded the empirical formula
, Z = 2, Dcalc. 3.041 g cm–3, from Lovozero alkaline massif, Kola Peninsula, Russia, has been solved and refined to R1 = 13.9% on the basis of 1745 unique reflections (Fo > 15σF). Electron microprobe analysis yielded the empirical formula 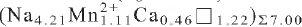
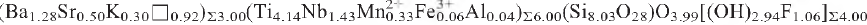 (H20)6.01, Z = 2, calculated on the basis of 42 (O + F) a.p.f.u., H2O and OH are calculated from structure refinement (H2O = 6 p.f.u.; F + OH = 4 p.f.u.). The crystal structure of nechelyustovite is a combination of a TS (titanium silicate) block and an I (intermediate) block. The TS block consists of HOH sheets (H-heteropolyhedral, O-octahedral). The TS block exhibits linkage and stereochemistry typical for Group III (Ti = 3 a.p.f.u.) of Ti-disilicate minerals: two H sheets connect to the O sheet such that two (Si2O7) groups link to the trans edges of a Ti octahedron of the O sheet. There are two distinct TS blocks of the same topology, TS1 and TS2, that differ in the cations of the O sheet, [(Na1.5Mn1☐0.5)Ti] and [(Na2Mn0.5☐0.5)Ti] (4 a.p.f.u.) respectively. The TS1 and TS2 blocks have two different H sheets, H1,2 and H3,4, where (Si2O7) groups link to [5]- and [6]-coordinated (Ti,Nb) polyhedra respectively. There are three peripheral sites, AP(1—3), occupied mainly by Ba (less Sr and K) at 96, 86 and 26% and one peripheral site AP(4) occupied by Na at 50%. There are two I blocks: the I1 block is a layer of Ba atoms; the I2 block consists of H2O groups and AP(3) atoms. TS blocks alternate with I blocks or link through hydrogen bonds (as in epistolite). There is a sequence of four TS blocks and three I blocks per the c cell parameter: TS2 — I1 — TS1 — I2 — TS1 — I1 — TS2.
(H20)6.01, Z = 2, calculated on the basis of 42 (O + F) a.p.f.u., H2O and OH are calculated from structure refinement (H2O = 6 p.f.u.; F + OH = 4 p.f.u.). The crystal structure of nechelyustovite is a combination of a TS (titanium silicate) block and an I (intermediate) block. The TS block consists of HOH sheets (H-heteropolyhedral, O-octahedral). The TS block exhibits linkage and stereochemistry typical for Group III (Ti = 3 a.p.f.u.) of Ti-disilicate minerals: two H sheets connect to the O sheet such that two (Si2O7) groups link to the trans edges of a Ti octahedron of the O sheet. There are two distinct TS blocks of the same topology, TS1 and TS2, that differ in the cations of the O sheet, [(Na1.5Mn1☐0.5)Ti] and [(Na2Mn0.5☐0.5)Ti] (4 a.p.f.u.) respectively. The TS1 and TS2 blocks have two different H sheets, H1,2 and H3,4, where (Si2O7) groups link to [5]- and [6]-coordinated (Ti,Nb) polyhedra respectively. There are three peripheral sites, AP(1—3), occupied mainly by Ba (less Sr and K) at 96, 86 and 26% and one peripheral site AP(4) occupied by Na at 50%. There are two I blocks: the I1 block is a layer of Ba atoms; the I2 block consists of H2O groups and AP(3) atoms. TS blocks alternate with I blocks or link through hydrogen bonds (as in epistolite). There is a sequence of four TS blocks and three I blocks per the c cell parameter: TS2 — I1 — TS1 — I2 — TS1 — I1 — TS2.
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2009
References
A correction has been issued for this article:
- 5
- Cited by
Linked content
Please note a has been issued for this article.




