Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Taylor, D.
1984.
The structural behaviour of tetrahedral framework compounds — a review Part II. Framework structures.
Mineralogical Magazine,
Vol. 48,
Issue. 346,
p.
65.
Al-Ajdah, Ghassan N. D.
Al-Rished, A. A.
Beagley, Brian
Dwyer, John
Fitch, Frank R.
and
Ibrahim, Talib K.
1985.
On the structures of X- and Y-type zeolites. Structural changes within the cavities of Na-X during dehydration.
Journal of Inclusion Phenomena,
Vol. 3,
Issue. 2,
p.
135.
Newsam, J.M.
and
Jorgensen, J.D.
1987.
Gallosilicate sodalite—further syntheses and structural details.
Zeolites,
Vol. 7,
Issue. 6,
p.
569.
Buckley, R. G.
Deckman, H. W.
Newsam, J. M.
McHenry, J. A.
Persans, P. D.
and
Witzke, H.
1987.
Structure-Sensitive Vibrations in Zeolites as Studied by Raman Scattering.
MRS Proceedings,
Vol. 111,
Issue. ,
Engelhardt, G.
Luger, S.
Buhl, J.Ch.
and
Felsche, J.
1989.
29Si MAS n.m.r. of aluminosilicate sodalites: Correlations between chemical shifts and structure parameters.
Zeolites,
Vol. 9,
Issue. 3,
p.
182.
Engelhardt, Günter
1989.
Recent Advances in Zeolite Science, Proceedings of the 1989 Meeting of the British Zeolite Association.
Vol. 52,
Issue. ,
p.
151.
Sharp, Z.D
Helffrich, G.R
Bohlen, S.R
and
Essene, E.J
1989.
The stability of sodalite in the system NaAlSiO4-NaCl.
Geochimica et Cosmochimica Acta,
Vol. 53,
Issue. 8,
p.
1943.
Jacobsen, H.S.
Norby, P.
Bildsøe, H.
and
Jakobsen, H.J.
1989.
1:1 Correlation between27Al and 29Si chemical shifts and correlations with lattice structures for some aluminosilicate sodalites.
Zeolites,
Vol. 9,
Issue. 6,
p.
491.
Nielsen, Niels Chr.
Bildsøe, Henrik
Jakobsen, Hans J.
and
Norby, Poul
1991.
7Li, 23Na, and 27Al quadrupolar interactions in some aluminosilicate sodalites from MAS n.m.r. spectra of satellite transitions.
Zeolites,
Vol. 11,
Issue. 6,
p.
622.
Beagley, Brian
and
Titiloye, James O.
1992.
Modeling the similarities and differences between the sodalite cages (?-cages) in the generic materials.
Structural Chemistry,
Vol. 3,
Issue. 6,
p.
429.
Stucky, Galen D.
1992.
Progress in Inorganic Chemistry.
Vol. 40,
Issue. ,
p.
99.
Titiloye, James O.
Tschaufeser, Petra
and
Parker, Stephen C.
1992.
Spectroscopic and Computational Studies of Supramolecular Systems.
Vol. 4,
Issue. ,
p.
137.
Srdanov, V.I.
Blake, N.P.
Markgraber, D.
Metiu, H.
and
Stucky, G.D.
1994.
Advanced Zeolite Science and Applications.
Vol. 85,
Issue. ,
p.
115.
Blake, Nick P.
and
Metiu, Horia
1995.
Efficient absorption line shape calculations for an electron coupled to many quantum degrees of freedom: Applications to an electron solvated in dry sodalite and halo-sodalites.
The Journal of Chemical Physics,
Vol. 103,
Issue. 11,
p.
4455.
Hong, Suk Bong
Camblor, Miguel A.
and
Davis, Mark E.
1997.
Host−Guest Interactions in Pure-Silica and Aluminosilicate Sodalites Containing Ethylene Glycol as a Guest Molecule.
Journal of the American Chemical Society,
Vol. 119,
Issue. 4,
p.
761.
Johnson, Geoffrey M.
and
Weller, Mark T.
1999.
A Powder Neutron Diffraction Study of Lithium-Substituted Gallosilicate and Aluminogermanate Halide Sodalites.
Inorganic Chemistry,
Vol. 38,
Issue. 10,
p.
2442.
Johnson, Geoffrey M
Mead, Philip J
and
Weller, Mark T
2000.
Synthesis of a range of anion-containing gallium and germanium sodalites.
Microporous and Mesoporous Materials,
Vol. 38,
Issue. 2-3,
p.
445.
Johnson, Geoffrey M.
Mead, Philip J.
Dann, Sandra E.
and
Weller, Mark T.
2000.
Multinuclear MAS NMR Studies of Sodalitic Framework Materials.
The Journal of Physical Chemistry B,
Vol. 104,
Issue. 7,
p.
1454.
Kendrick, Emma
and
Dann, Sandra
2004.
Synthesis, properties and structure of ion exchanged hydrosodalite.
Journal of Solid State Chemistry,
Vol. 177,
Issue. 4-5,
p.
1513.
Villars, P.
Cenzual, K.
Daams, J.
Gladyshevskii, R.
Shcherban, O.
Dubenskyy, V.
Melnichenko-Koblyuk, N.
Pavlyuk, O.
Stoiko, S.
and
Sysa, L.
2005.
Structure Types. Part 2: Space Groups (218) P-43n - (195) P23.
Vol. 43A2,
Issue. ,
p.
1.
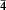 3n, but the NaI-sodalite fitted 1
3n, but the NaI-sodalite fitted 1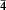 3m better. The resulting structural data reveal shortcomings in the previous computer models for sodalite structures and an improved computer modelling procedure is devised which successfully predicts atomic coordinates, starting from the experimental a value and an estimate of the cationanion distance. The method incorporates the experimental result that the average T-O distance (T = Al, Si) throughout the samples is ∼ 1.678 Å, and Si-O and Al-O are set at 1.618 and 1.738 Å, respectively. Although T-O remains little changed throughout the samples, the data confirm the inverse relationship between ∠ T-O-T and the tetrahedron tilt angle ϕ, in which ∠ T-O-T approaches ∼ 160° as ϕ → 0° and the sodalite cage becomes fully expanded.
3m better. The resulting structural data reveal shortcomings in the previous computer models for sodalite structures and an improved computer modelling procedure is devised which successfully predicts atomic coordinates, starting from the experimental a value and an estimate of the cationanion distance. The method incorporates the experimental result that the average T-O distance (T = Al, Si) throughout the samples is ∼ 1.678 Å, and Si-O and Al-O are set at 1.618 and 1.738 Å, respectively. Although T-O remains little changed throughout the samples, the data confirm the inverse relationship between ∠ T-O-T and the tetrahedron tilt angle ϕ, in which ∠ T-O-T approaches ∼ 160° as ϕ → 0° and the sodalite cage becomes fully expanded.



