Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Kim, Jangsuk
Simon, Arleyn W
Ripoche, Vincent
Mayer, James W
and
Wilkens, Barry
2003.
Proton-induced x-ray emission analysis of turquoise artefacts from Salado Platform Mound sites in the Tonto Basin of central Arizona.
Measurement Science and Technology,
Vol. 14,
Issue. 9,
p.
1579.
Crespo-Feo, E.
Garcia-Guinea, J.
Correcher, V.
and
Prado-Herrero, P.
2010.
Luminescence behavior of turquoise [CuAl6(PO4)4(OH)8·4H2O].
Radiation Measurements,
Vol. 45,
Issue. 3-6,
p.
749.
Welch, Mark D.
Sciberras, Matthew J.
Williams, Peter A.
Leverett, Peter
Schlüter, Jochen
and
Malcherek, Thomas
2014.
A temperature-induced reversible transformation between paratacamite and herbertsmithite.
Physics and Chemistry of Minerals,
Vol. 41,
Issue. 1,
p.
33.
Čejka, Jiří
Sejkora, Jiří
Macek, Ivo
Malíková, Radana
Wang, Lina
Scholz, Ricardo
Xi, Yunfei
and
Frost, Ray L.
2015.
Raman and infrared spectroscopic study of turquoise minerals.
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy,
Vol. 149,
Issue. ,
p.
173.
Laha, Sourav
Tamilarasan, Subramani
Natarajan, Srinivasan
and
Gopalakrishnan, Jagannatha
2016.
Stabilization of a Tetrahedral (Mn5+O4) Chromophore in Ternary Barium Oxides as a Strategy toward Development of New Turquoise/Green-Colored Pigments.
Inorganic Chemistry,
Vol. 55,
Issue. 7,
p.
3508.
Elliott, Peter
2017.
Refinement of the crystal structure of sieleckiite and revision
of its symmetry.
Mineralogical Magazine,
Vol. 81,
Issue. 4,
p.
917.
Rossi, Manuela
Rizzi, Rosanna
Vergara, Alessandro
Capitelli, Francesco
Altomare, Angela
Bellatreccia, Fabio
Saviano, Michele
and
Ghiara, Rosaria M.
2017.
Compositional variation of turquoise-group minerals from the historical collection of the Real Museo Mineralogico of the University of Naples.
Mineralogical Magazine,
Vol. 81,
Issue. 6,
p.
1405.
Sabbaghi, Hamid
2018.
A combinative technique to recognise and discriminate turquoise stone.
Vibrational Spectroscopy,
Vol. 99,
Issue. ,
p.
93.
Queffelec, Alain
2020.
Provenancing turquoise: a difficult task.
ArchéoSciences,
p.
19.
Dumańska‐Słowik, Magdalena
Wesełucha‐Birczyńska, Aleksandra
Natkaniec‐Nowak, Lucyna
Gaweł, Adam
Włodek, Adam
and
Kulmaczewska, Katarzyna
2020.
Blue or green? turquoise–planerite species from Carico Lake Valley in Nevada, the United States: Evidence from Raman spectroscopy.
Journal of Raman Spectroscopy,
Vol. 51,
Issue. 2,
p.
346.
Gandomani, Elahe Mansouri
Rashidnejad-Omran, Nematollah
Emamjomeh, Amir
Vignola, Pietro
and
Hashemzadeh, Tahereh
2020.
Electron microprobe study of turquoise-group solid solutions in the Neyshabour and Meydook mines, northeast and southern Iran.
The Canadian Mineralogist,
Vol. 58,
Issue. 1,
p.
71.
Wang, Xueding
and
Guo, Ying
2021.
The impact of trace metal cations and absorbed water on colour transition of turquoise.
Royal Society Open Science,
Vol. 8,
Issue. 2,
Nesheva, Larisa
Yanakieva, Denka
Petrov, Petko
Atanasova-Vladimirova, Stela
Petrova, Nadia
Vassileva, Rossitsa
Sergeeva, Ivanina
Cherkezova-Zheleva, Zara
and
Paneva, Daniela
2023.
Planerite with aheylite and faustite from the Chala deposit, Spahievo Ore Field.
Review of the Bulgarian Geological Society,
Vol. 84,
Issue. 3,
p.
43.
Yakubovich, Olga
Kiriukhina, Galina
Volkov, Anatoly
Simonov, Sergey
Yapaskurt, Vasiliy
and
Dimitrova, Olga
2024.
Crystal chemistry and predicted ionic conductivity of hydrothermally synthesized Na9Zn2(PO4)4Cl.
CrystEngComm,
Vol. 26,
Issue. 6,
p.
848.
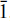 , with a = 7.419(2) [turquoise: 7.410(1)], b = 7.629(3) [7.633(1)], c = 9.905(3) [9.904(1)] Å, α = 69.17(2) [68.42(1)], β = 69.88(2) [69.65(1)], γ = 64.98(2) [65.05(1)]°, V = 462.2(3) [460.27(10)] Å3, and Z = 1. The structure consists of distorted MO6 polyhedra (M = Zn, Cu), AlO6 octahedra and PO4 tetrahedra. By edge- and corner-sharing of these polyhedra a fairly dense three-dimensional framework is formed which is further strengthened by a system of hydrogen bonds. The metal atoms in the unique MO6 (M = Zn or Cu) polyhedron show a distorted [2+2+2]-coordination, the distortion being more pronounced in turquoise. About 10% of the M site is vacant in both minerals. In turquoise, a previously undetected structural site with a very low occupancy of (possibly) Cu is present at the position (Ý,0,Ý).
, with a = 7.419(2) [turquoise: 7.410(1)], b = 7.629(3) [7.633(1)], c = 9.905(3) [9.904(1)] Å, α = 69.17(2) [68.42(1)], β = 69.88(2) [69.65(1)], γ = 64.98(2) [65.05(1)]°, V = 462.2(3) [460.27(10)] Å3, and Z = 1. The structure consists of distorted MO6 polyhedra (M = Zn, Cu), AlO6 octahedra and PO4 tetrahedra. By edge- and corner-sharing of these polyhedra a fairly dense three-dimensional framework is formed which is further strengthened by a system of hydrogen bonds. The metal atoms in the unique MO6 (M = Zn or Cu) polyhedron show a distorted [2+2+2]-coordination, the distortion being more pronounced in turquoise. About 10% of the M site is vacant in both minerals. In turquoise, a previously undetected structural site with a very low occupancy of (possibly) Cu is present at the position (Ý,0,Ý).



