Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Breza, Martin
and
Biskupič, Stanislav
2004.
On the Structure of Boat-Shaped Hexalead(II) Cations with OH Bridges.
Collection of Czechoslovak Chemical Communications,
Vol. 69,
Issue. 11,
p.
2055.
Breza, Martin
and
Manová, Alena
2006.
On the bridging mode in tetrahedral tetralead(II) hydroxocomplexes.
Journal of Molecular Structure: THEOCHEM,
Vol. 765,
Issue. 1-3,
p.
121.
Siidra, Oleg I.
Krivovichev, Sergey V.
and
Filatov, Stanislav K.
2008.
Minerals and synthetic Pb(II) compounds with oxocentered tetrahedra: review and classification.
Zeitschrift für Kristallographie - Crystalline Materials,
Vol. 223,
Issue. 1-2,
p.
114.
Mauck, Catherine M.
van den Heuvel, Titus W. P.
Hull, Michaela M.
Zeller, Matthias
and
Oertel, Catherine M.
2010.
Synthesis and Structures of Pb3O2(CH3COO)2·0.5H2O and Pb2O(HCOO)2: Two Corrosion Products Revisited.
Inorganic Chemistry,
Vol. 49,
Issue. 22,
p.
10736.
Krivovichev, Sergey V.
Mentré, Olivier
Siidra, Oleg I.
Colmont, Marie
and
Filatov, Stanislav K.
2013.
Anion-Centered Tetrahedra in Inorganic Compounds.
Chemical Reviews,
Vol. 113,
Issue. 8,
p.
6459.
Wang, Genxiang
Luo, Min
Lin, Chensheng
Ye, Ning
Zhou, Yuqiao
and
Cheng, Wendan
2014.
Lanthanum Lead Oxide Hydroxide Nitrates with a Nonlinear Optical Effect.
Inorganic Chemistry,
Vol. 53,
Issue. 23,
p.
12584.
Wang, Genxiang
Luo, Min
Ye, Ning
Lin, Chensheng
and
Cheng, Wendan
2014.
Series of Lead Oxide Hydroxide Nitrates Obtained by Adjusting the pH Values of the Reaction Systems.
Inorganic Chemistry,
Vol. 53,
Issue. 10,
p.
5222.
Diana, Eliano
Gervasio, Giuliana
Priola, Emanuele
and
Bonometti, Elisabetta
2014.
A new heterometallic multiligand 3D coordination polymer: synthesis and structure of [Pb(OH)]n[Ag(SCN)(CN)]n.
CrystEngComm,
Vol. 16,
Issue. 43,
p.
10040.
Chukanov, Nikita V.
Jonsson, Erik
Aksenov, Sergey M.
Britvin, Sergey N.
Rastsvetaeva, Ramiza K.
Belakovskiy, Dmitriy I.
and
Van, Konstantin V.
2017.
Roymillerite, Pb24Mg9(Si9AlO28)(SiO4)(BO3)(CO3)10(OH)14O4, a new mineral: mineralogical characterization and crystal chemistry.
Physics and Chemistry of Minerals,
Vol. 44,
Issue. 10,
p.
685.
Britvin, S. N.
Pekov, I. V.
Yapaskurt, V. O.
Koshlyakova, N. N.
Göttlicher, J.
Krivovichev, S. V.
Turchkova, A. G.
and
Sidorov, E. G.
2020.
Polyoxometalate chemistry at volcanoes: discovery of a novel class of polyoxocuprate nanoclusters in fumarolic minerals.
Scientific Reports,
Vol. 10,
Issue. 1,
Firmansyah
Kartini, I
Sutarno
and
Ruslan
2021.
Effect of additive polarity on the stability of organic-inorganic hybrid CH3NH3PbI3 perovskite thin film.
Journal of Physics: Conference Series,
Vol. 1763,
Issue. 1,
p.
012032.
Yang, Peng
and
Kortz, Ulrich
2021.
Comprehensive Coordination Chemistry III.
p.
4.
Kampf, Anthony R.
Hughes, John M.
Nash, Barbara P.
and
Marty, Joe
2022.
Nitroplumbite, [Pb4(OH)4](NO3)4, a New Mineral with Cubane-Like [Pb4(OH)4]4+ Clusters from the Burro Mine, San Miguel County, Colorado, USA.
The Canadian Mineralogist,
Vol. 60,
Issue. 5,
p.
787.
Kampf, Anthony R.
Harlow, George E.
and
Ma, Chi
2023.
Pohlite, a new lead iodate hydroxide chloride from Sierra Gorda, Chile.
Mineralogical Magazine,
Vol. 87,
Issue. 2,
p.
171.
Kampf, Anthony R.
Smith, Jason B.
Hughes, John M.
Ma, Chi
and
Emproto, Christopher
2023.
New Minerals from the Redmond Mine, North Carolina, USA: II. Cubothioplumbite and Hexathioplumbite, Two New Minerals Containing the Cubane-Like [Pb4(OH)4]4+ Structural Unit.
The Canadian Journal of Mineralogy and Petrology,
Vol. 61,
Issue. 3,
p.
623.
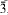 , with a = 10.263(1), c = 25.454(5) Å, and a structure solution is in complete agreement with an independent single-crystal study by Li et al. (2001). Probable hydrogen bonds in Pb2(OH)3(NO3) are indicated. Reported data on
, with a = 10.263(1), c = 25.454(5) Å, and a structure solution is in complete agreement with an independent single-crystal study by Li et al. (2001). Probable hydrogen bonds in Pb2(OH)3(NO3) are indicated. Reported data on 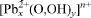 clusters and lead hydroxide and oxide nitrates are summarized and discussed critically. The probable conditions of formation of the studied samples are evaluated.
clusters and lead hydroxide and oxide nitrates are summarized and discussed critically. The probable conditions of formation of the studied samples are evaluated.



