Article contents
Crystal structure and crystal chemistry of fluoro-potassic-magnesio-arfvedsonite from Monte Metocha, Xixano region, Mozambique, and discussion of the holotype from Quebec, Canada
Published online by Cambridge University Press: 05 July 2018
Abstract
Fluoro-potassic-magnesio-arfvedsonite, ideally AKBNa2c(Mg4Fe3+)TSi8O22wF2, has been found in a dyke ∼25 km southwest of Monte Metocha, Xixano region, northeastern Mozambique. Fluoro-potassic-magnesio-arfvedsonite and low sanidine form a fine-grained mafic, ultrapotassic, peralkaline igneous rock without visible phenocrysts. The amphibole is brittle, has a Mohs hardness of 6 and a splintery fracture; it is non-fluorescent with perfect {110} cleavage and no observable parting, and has a calculated density of 3.174 gcm−3. In plane-polarized light, it is pleochroic, X= pale grey-green, Y = blue-green, Z = pale grey; X ^ c = 23.6° (in β obtuse), Y ‖ b, Z ^ c = 66.4° (in β acute). Fluoro-potassic-magnesio-arfvedsonite is biaxial negative, α = 1.652(2), β = 1.658(2), γ = 1.660(2); 2Vobs = 22.5(7)°, 2Vcalc = 30.2°. The unit-cell dimensions are a = 9.9591(4), b = 17.9529(7), c = 5.2867(2) Å, β = 104.340(1)°, V = 919.73(10) Å3, Z = 2. The nine strongest X-ray diffraction lines in the experimental powder pattern are: [d in Å(I)(hkl)]: 2.716(100)(151), 3.410(70)(131), 8.475(50)(110), 3.178(50)(310), 3.309(30)(240), 2.762(20)(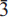 31), 2.549(20)(260), 2.351(10)(
31), 2.549(20)(260), 2.351(10)(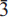 51), 2.269(10)(331). Electron microprobe analysis gave: SiO2 54.25, A12O3 0.03, TiO2 1.08, FeO 6.69, Fe2O3 8.07, MgO 13.99, MnO 0.32, ZnO 0.05, CaO 1.16, Na2O 6.33, K2O 5.20, F 2.20, H2Ocalc 0.74, sum 99.18 wt.%. The formula unit, calculated on the basis of 24 (O,OH,F) with (OH+F) = 2−(2 Ti), is AKa0.98B(Na1.18Ca0.18)∑1.99C(Mg3.07Fe0.832+Mn0.04Al0.01Fe0.903+Ti0.12Zn0.01)∑=4.98TSi8O22W[Fi.o3(OH)0.73O0.24]∑2.00 and confirms the usual pattern of cation order in the amphibole structure. The presence of a significant oxo component (locally balanced by Ti at the M(1) site) is related to the crystallization conditions. The presence of Fe3+ at the T sites, originally suggested for the holotype specimen, is discounted for this amphibole composition.
51), 2.269(10)(331). Electron microprobe analysis gave: SiO2 54.25, A12O3 0.03, TiO2 1.08, FeO 6.69, Fe2O3 8.07, MgO 13.99, MnO 0.32, ZnO 0.05, CaO 1.16, Na2O 6.33, K2O 5.20, F 2.20, H2Ocalc 0.74, sum 99.18 wt.%. The formula unit, calculated on the basis of 24 (O,OH,F) with (OH+F) = 2−(2 Ti), is AKa0.98B(Na1.18Ca0.18)∑1.99C(Mg3.07Fe0.832+Mn0.04Al0.01Fe0.903+Ti0.12Zn0.01)∑=4.98TSi8O22W[Fi.o3(OH)0.73O0.24]∑2.00 and confirms the usual pattern of cation order in the amphibole structure. The presence of a significant oxo component (locally balanced by Ti at the M(1) site) is related to the crystallization conditions. The presence of Fe3+ at the T sites, originally suggested for the holotype specimen, is discounted for this amphibole composition.
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2010
References
- 7
- Cited by




