Article contents
Cámaraite, Ba3NaTi4(Fe2+,Mn)8(Si2O7)4O4(OH,F)7. I. A new Ti-silicate mineral from the Verkhnee Espe Deposit, Akjailyautas Mountains, Kazakhstan
Published online by Cambridge University Press: 05 July 2018
Abstract
Cámaraite, ideally Ba3NaTi4(Fe2+,Mn)8(Si2O7)4O4(OH,F)7, is a new mineral from the Verkhnee Espe deposit, Akjailyautas Mountains, Kazakhstan. It occurs as intergrowths with bafertisite and jinshajiangite in separate platy crystals up to 8 mm × 15 mm × 2 mm in size, or as star-shaped aggregates of crystals with different orientations. Individual crystals are orange-red to brownish-red, and are platy on {001}. Cámaraite is translucent and has a pale-yellow streak, a vitreous lustre, and does not fluoresce under cathode or ultraviolet light. Cleavage is {001} perfect, no parting was observed, and Mohs hardness is <5; the mineral is brittle. The calculated density is 4.018 g cm-3. In transmitted light, camaraite is strongly pleochroic, X = light brown, Y = reddish-brown, Z = yellow- brown, with Z < X < Y. Cámaraite is biaxial +ve and 2Vmeas. = 93(1)°. All refractive indices are greater than 1.80. Cámaraite is triclinic, space group C , a = 10.678(4) Å, b = 13.744(8) Å, c = 21.40(2) Å, α = 99.28(8)°, β = 92.38(5)°, γ = 90.00(6)°, V = 3096(3) Å3, Z = 4, a:b:c = 0.7761:1:1.5565. The seven strongest lines in the X-ray powder-diffraction pattern are as follows: [d (Å), (I), (hkl)]: 2.63, (100), (401); 2.79, (90), (
, a = 10.678(4) Å, b = 13.744(8) Å, c = 21.40(2) Å, α = 99.28(8)°, β = 92.38(5)°, γ = 90.00(6)°, V = 3096(3) Å3, Z = 4, a:b:c = 0.7761:1:1.5565. The seven strongest lines in the X-ray powder-diffraction pattern are as follows: [d (Å), (I), (hkl)]: 2.63, (100), (401); 2.79, (90), (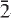
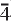 3,
3, 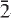 41, 2
41, 2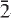 6, 225); 1.721, (70), (
6, 225); 1.721, (70), (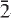
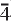 11,
11, 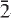 49, 0
49, 0 2); 3.39, (50), (2
2); 3.39, (50), (2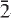 4, 223); 3.18, (50), (
4, 223); 3.18, (50), (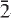
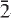 5,
5, 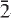 24); 2.101, (50), (
24); 2.101, (50), (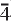
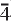 2,
2, 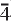 40); 1.578, (50), (
40); 1.578, (50), (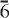
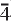 1,
1, 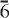
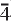 2, 6
2, 6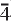 1,
1, 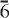 40). Chemical analysis by electron microprobe gave: Nb2O5 1.57, SiO2 25.25, TiO2 15.69, ZrO2 0.33, Al2O3 0.13, Fe2O3 2.77, FeO 16.54, MnO 9.46, ZnO 0.12, MgO 0.21, CaO 0.56, BaO 21.11, Na2O 1.41, K2O 0.84, H2O 1.84, F 3.11, less O:F 1.31, total 99.63 wt.%, where the valence state of Fe was determined by Mössbauer spectroscopy [Fe3+/(Fe2+ + Fe3+) = 0.13(8)] and the H2O content was derived by crystal-structure determination. The resulting empirical formula on the basis of 39 anions is
40). Chemical analysis by electron microprobe gave: Nb2O5 1.57, SiO2 25.25, TiO2 15.69, ZrO2 0.33, Al2O3 0.13, Fe2O3 2.77, FeO 16.54, MnO 9.46, ZnO 0.12, MgO 0.21, CaO 0.56, BaO 21.11, Na2O 1.41, K2O 0.84, H2O 1.84, F 3.11, less O:F 1.31, total 99.63 wt.%, where the valence state of Fe was determined by Mössbauer spectroscopy [Fe3+/(Fe2+ + Fe3+) = 0.13(8)] and the H2O content was derived by crystal-structure determination. The resulting empirical formula on the basis of 39 anions is 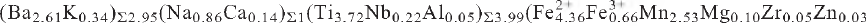 Ca0.05)Σ7.78Si7.97O35.89H3.88F3.11. Cámaraite is a Group-II TS-block mineral in the structure hierarchy of Sokolova (2006). The mineral is named camaraite after Fernando Cámaraite (born 1967) of Melilla, Spain, in recognition of his contribution to the fields of mineralogy and crystallography. The new mineral and mineral name have been approved by the Commission on New Minerals, Nomenclature and Classification, International Mineralogical Association (IMA 2009-11).
Ca0.05)Σ7.78Si7.97O35.89H3.88F3.11. Cámaraite is a Group-II TS-block mineral in the structure hierarchy of Sokolova (2006). The mineral is named camaraite after Fernando Cámaraite (born 1967) of Melilla, Spain, in recognition of his contribution to the fields of mineralogy and crystallography. The new mineral and mineral name have been approved by the Commission on New Minerals, Nomenclature and Classification, International Mineralogical Association (IMA 2009-11).
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2009
References
- 16
- Cited by




