No CrossRef data available.
Article contents
The Relevance of Ultrastructural Studies of Metastatic Cells from Women with Breast Cancer History
Published online by Cambridge University Press: 17 November 2021
Abstract
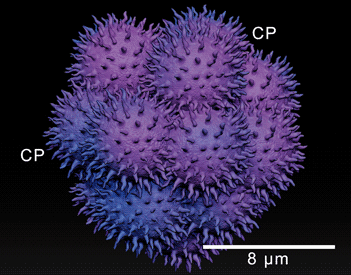
The interaction between cancer cells and the surrounding microenvironment is determinant for metastasis success. In this study, the ultrastructural relevance of cells in the malignant pleural effusion (MPE) of women with breast cancer history was investigated. In MPE, it is possible to observe single cells and clusters. Women whose MPE presents carcinomas in aggregates have a better prognosis when compared to cases in which metastatic single cells are found. Samples were collected via fine-needle aspiration puncture (US-FNA). Subsequent to the material preparation and ultrathin cuts, they were observed using light and transmission electron microscopy (LM/TEM). LM and TEM images served as a basis for the creation of a digital sculpture using ZBrush® software. Clusters exhibited structural stability, en route vesicles allowing exocytosis of electron-dense fibrous elements, and cytoplasmic protrusions contributing to migratory and invasive skills. Single cells presented different necrotic phenotypes and many displayed leukocyte-like characteristics. Cluster cooperative relationships seem to be related to a long-term permanence in MPE. The absence of a collaborative network presumably triggers a more aggressive behavior of single cells. Its putative fusion with leukocytes can maximize the efficiency for transendothelial migration, increasing chances of metastatic success and, unfortunately, reducing survival of women with recidivism.
Keywords
- Type
- Biological Applications
- Information
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press on behalf of the Microscopy Society of America





