Article contents
Immunofluorescence Image Feature Analysis and Phenotype Scoring Pipeline for Distinguishing Epithelial–Mesenchymal Transition
Published online by Cambridge University Press: 20 May 2021
Abstract
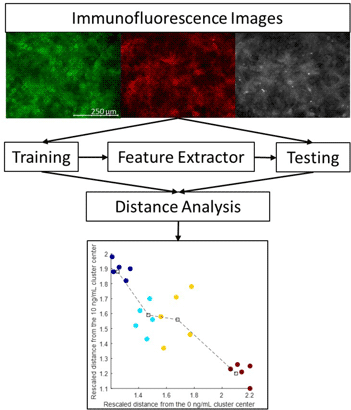
Epithelial–mesenchymal transition (EMT) is an essential biological process, also implicated in pathological settings such as cancer metastasis, in which epithelial cells transdifferentiate into mesenchymal cells. We devised an image analysis pipeline to distinguish between tissues comprised of epithelial and mesenchymal cells, based on extracted features from immunofluorescence images of differing biochemical markers. Mammary epithelial cells were cultured with 0 (control), 2, 4, or 10 ng/mL TGF-β1, a well-established EMT-inducer. Cells were fixed, stained, and imaged for E-cadherin, actin, fibronectin, and nuclei via immunofluorescence microscopy. Feature selection was performed on different combinations of individual cell markers using a Bag-of-Features extraction. Control and high-dose images comprised the training data set, and the intermediate dose images comprised the testing data set. A feature distance analysis was performed to quantify differences between the treatment groups. The pipeline was successful in distinguishing between control (epithelial) and the high-dose (mesenchymal) groups, as well as demonstrating progress along the EMT process in the intermediate dose groups. Validation using quantitative PCR (qPCR) demonstrated that biomarker expression measurements were well-correlated with the feature distance analysis. Overall, we identified image pipeline characteristics for feature extraction and quantification of immunofluorescence images to distinguish progression of EMT.
- Type
- Biological Applications
- Information
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press on behalf of the Microscopy Society of America
References
- 5
- Cited by





