No CrossRef data available.
Article contents
Age-Related Changes in Calcitonin-Producing Thyroid C-Cells of Male Wistar Rats
Published online by Cambridge University Press: 20 May 2022
Abstract
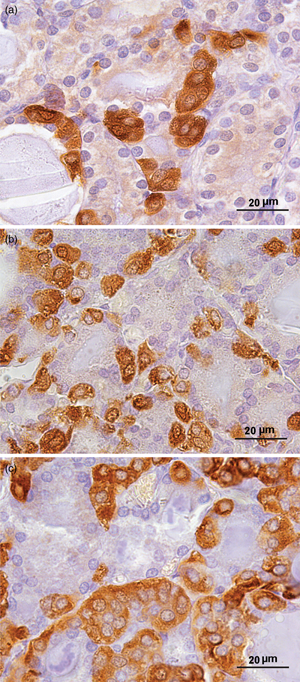
Thyroid C-cells secrete the hormone calcitonin (CT) which acts as an inhibitor of bone resorption. Our aim was to examine the age-related changes in the structure and function of CT-producing C-cells, using histomorphometric, ultrastructural, and biochemical analyses. We used young adult (3-months-old), middle-aged (16-months-old), and old (24-months-old) male rats. The peroxidase-antiperoxidase method was applied for localization of CT. Stereological analysis was performed using the newCAST stereological software package. Serum samples were analyzed for the determination of CT, testosterone (T), calcium (Ca2+), and phosphorus (P). We found a significant increase in the volume density (Vv) of C-cells in both older groups (p < 0.05). The percentage of smaller volume range C-cells increased (p < 0.0001), while the proportion of greater volume range C-cells decreased (p < 0.05) with ageing. Ultrastructural analysis revealed a larger number of secretory granules in older rats. Serum CT increased (p < 0.001), while serum T and P were reduced (p < 0.01) in older rats. Serum Ca2+ was lower (p < 0.0001) in middle-aged rats compared to young adults. We revealed a 20% incidence of C-cell hyperplasia in older rats and one case of medullary thyroid carcinoma in an old rat. Our findings indicate that the ageing process causes significant histomorphometric changes at the thyroid C-cell level.
- Type
- Biological Applications
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press on behalf of the Microscopy Society of America




