Article contents
Lattice defects in LiCoO2
Published online by Cambridge University Press: 02 July 2020
Abstract
Rechargeable Lithium ion batteries are widely used as portable power source in communication and computer technology, prospective uses include medical implantable devices and electric vehicles. The safety and cycle life of Li ion batteries is improved over that of batteries containing metallic lithium anodes because the insertion of Li between the crystal layers of both electrodes was proved to be safer than the electroplating of Li onto a metallic Lithium anode. in Li-ion batteries, the charge transport is governed by the oscillation of Li ions between anode and cathode. They are sometimes called “rocking-chair“ batteries. The most common materials for these batteries are lithiated carbons for anodes, and transition metal oxides (LixCoO2) as cathodes.
LixCoO2 has an ordered rhombohedral R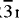 m structure consisting of alternating layers of Co-O-Li-O-Co. The capacity and energy density of the batteries is limited by the amount of Li that can be stored in the anode and cathode materials.
m structure consisting of alternating layers of Co-O-Li-O-Co. The capacity and energy density of the batteries is limited by the amount of Li that can be stored in the anode and cathode materials.
- Type
- Microscopy in the Real World: Semiconductors and Materials
- Information
- Copyright
- Copyright © Microscopy Society of America 2001
References
[1] Reimers, J.N. and Dahn, J.R., J. Electrochemical Society, Vol. 139, No. 8, 2091–2096,(1992)CrossRefGoogle Scholar
[2] Ohzuku, T. and Ueda, A., J. Electrochemical Society, Vol.141, No. 11, 2972–2977, (1994)CrossRefGoogle Scholar
[3] Wang, H., Jang, Y.-I., Huang, B., Sadoway, D.R., Chiang, Y.-M., J. Power Sources, 81-21,595–598,(1999)Google Scholar
- 1
- Cited by




