Introduction
Roads unavoidably remove habitat available to fauna by linear gaps (Clevenger Reference Clevenger2005; Hawbaker et al. Reference Hawbaker, Radeloff, Clayton, Hammer and Gonzalez-Abraham2006; Miller et al. Reference Miller, Joyce, Knight and King1996; Perz et al. Reference Perz, Caldas, Walker, Arima and Souza2008; Roedenbeck et al. Reference Roedenbeck, Fahrig, Findlay, Houlahan, Jaeger, Klar, Kramer-Schadt and van der Grift2007) and act as barriers to animal movement or alter movement patterns such as home range, daily movement, or migration (Brody & Pelton Reference Brody and Pelton1989; Gibson & Koenig Reference Gibson and Koenig2012; Ortega & Capen Reference Ortega and Capen1999; Trombulak & Frissell Reference Trombulak and Frissell2000). Animals are often reluctant to approach roads because of the risk of mortality by vehicle collision or simply due to noise, smell, or fear of humans (Forman & Alexander Reference Forman and Alexander1998; Goosem & Marsh Reference Goosem and Marsh1997; Jaeger et al. Reference Jaeger, Bowman, Brennan, Fahrig, Bert, Bouchard, Charbonneau, Frank, Gruber and von Toschanowitz2005). These impacts may generate spatial impediments that restrict reproduction, feeding opportunities, and gene flow, and in turn create decline in species population (Andren,Reference Andren1994; Hawbaker et al. Reference Hawbaker, Radeloff, Clayton, Hammer and Gonzalez-Abraham2006; Riley et al. Reference Riley, Pollinger, Sauvajot, York, Bromley, Fuller and Wayne2006; Saunders et al. Reference Saunders, Hobbs and Margules1991). Therefore, understanding the way animals behave under the presence of roads is fundamental to mitigate their inherently negative effects (Chen & Koprowski Reference Chen and Koprowski2016).
While aforementioned studies deal with the impact of major roads with high traffic volume (e.g. two multilane paved roadways separated by a median) on animal communities (Bennett Reference Bennett2017), roads in protected areas have received less attention (Laurance et al. Reference Laurance, Stouffer and Laurance2004). Protected area managers are particularly aware of the impact of roads on flora and fauna, and consequently follow guidelines to lay roads in a way that they are mitigated to the maximum (Andrews et al. Reference Andrews, Nanjappa and Riley2015; Boston Reference Boston2016; Sessions Reference Sessions2007; Transportation Research Board and National Research Council 2005). Accordingly, roads in protected areas are usually narrow and protected from heavy traffic to minimise their impact on flora and fauna. Despite this effort, some damage to the forest habitat at the roadsides of protected areas is unavoidable (Estrada et al. Reference Estrada, Garber, Rylands, Roos, Fernandez-Duque, Di Fiore, Nekaris, Nijman, Heymann, Lambert, Rovero, Barelli, Setchell, Gillespie, Mittermeier, Arregoitia, de Guinea, Gouveia, Dobrovolski, Shanee, Shanee, Boyle, Fuentes, MacKinnon, Amato, Meyer, Wich, Sussman, Pan, Kone and Li2017; Laurance et al. Reference Laurance, Goosem and Laurance2009), even along narrow dirt roads (Laurance et al. Reference Laurance, Stouffer and Laurance2004). Besides, the mere presence of any type of road in protected areas gives potential access to poachers who capture, kill, and butcher wild animals (Beringer et al. Reference Beringer, Seibert and Pelton1990), which consequently constitutes hot zones where pathogens may jump to people (Wolfe et al. Reference Wolfe, Daszak, Kilpatrick and Burke2005).
Roads can critically break tree canopy connectivity and alter edge habitat, making a real barrier to the movement of tree dwellers, particularly if animals have difficulties to use the unfamiliar road substrate (Cannon & Leighton Reference Cannon and Leighton1994; Cristóbal-Azkarate & Arroyo-Rodríguez Reference Cristóbal-Azkarate and Arroyo-Rodríguez2007; Laurance Reference Laurance, Lowman, Devy and Ganesh2013; Milton Reference Milton, Boinski and Garber2000; Thorpe et al. Reference Thorpe, Crompton and Alexander2007; Wilson et al. Reference Wilson, Marsh and Winter2007). A wide canopy gap is the primary obstacle imposed by roads to the movement of arboreal mammals (Asari et al. Reference Asari, Johnson, Parsons and Lason2010; Cheyne et al. Reference Cheyne, Thompson and Chivers2013; van der Ree Reference van der Ree2002). That is, there is a greater road permeability to arboreal individuals where the distances necessary to cross roads using tree branches are shorter. Accordingly, roads can also outline the edges of home ranges in arboreal taxa at those road segments with large gaps across both sides (Asensio et al. Reference Asensio, Murillo-Chacon, Schaffner and Aureli2017).
Gibbons of the genus Hylobates are small apes that live in small family groups consisting of an adult mating pair and offspring (Bartlett Reference Bartlett2009; Brockelman Reference Brockelman, Lappan and Whittaker2009; Leighton Reference Leighton, Smuts, Cheney, Seyfarth, Wrangham and Struhsaker1987; Reichard Reference Reichard, Reichard and Boesch2003). Gibbons live in relatively small and highly stable territories across years (ca 25–40 ha), which they quickly traverse on a daily basis (Asensio et al. Reference Asensio, Murillo-Chacon, Schaffner and Aureli2017; Bartlett Reference Bartlett2009; Bartlett et al. Reference Bartlett, Light and Brockelman2015; Cheyne et al. Reference Cheyne, Capilla, Supiansyah, Cahyaningrum and Smith2019). Gibbons constitute suitable models to investigate how roads affect the movement of arboreal animals given its strict tree lifestyle and dependence on areas of quality forest (Carpenter Reference Carpenter and Rumbaugh1972; Meijaard et al., Reference Meijaard, Sheil, Nasi, Augeri, Rosenbaum, Iskandar and Soehartono2005; Oka et al. Reference Oka, Iskandar, Ghozali, Guhardja, Fatawi, Sutisna, Mori and Ohta2000; Syxaiyakhamthor et al. Reference Syxaiyakhamthor, Ngoprasert, Asensio and Savini2019). Here, we investigated the effect of roads on the movement of gibbons (Hylobates lar and H. pileatus) in Khao Yai National Park, Thailand, by studying two groups whose home ranges included roads therein (roadside groups) and two groups living relatively close (around 500 m) to roads (interior groups). First, we compared home range characteristics between roadside and interior groups. Second, we estimated whether gibbons avoided being next to roads by studying whether space use decreased as they approached roads. To further investigate this avoidance, we tested whether the two roadside groups would have moved differently in the hypothetical absence of roads. Third, we examined which were the factors that determined road-crossing probability using generalised linear models (GLMs) through the characteristics of crossed and not crossed locations. Finally, we projected a road crossing probability map for gibbons for the entire park using the best GLMs as our model system.
Methods
Study area
Khao Yai National Park (2169 km2; 101°220 E, 14°260 N; Figure 1) contains areas of evergreen forest, hill evergreen forest, mixed deciduous forest, escarpment forest, hill evergreen forest, grasslands, and patches of fast regenerating forest at elevations of 400–1350 m above mean sea level (Brockelman et al. Reference Brockelman, Nathalang and Maxwel2017; Trisurat et al. Reference Trisurat, Eiumnoh, Murai, Hussain and Shrestha2000). A network of paved roads of about 6 m width, comprising a total of 83 km length, connects the north and south park gates, sightseeing sites, camping sites, and touristic and staff housing areas placed around park headquarters (Figure 1).
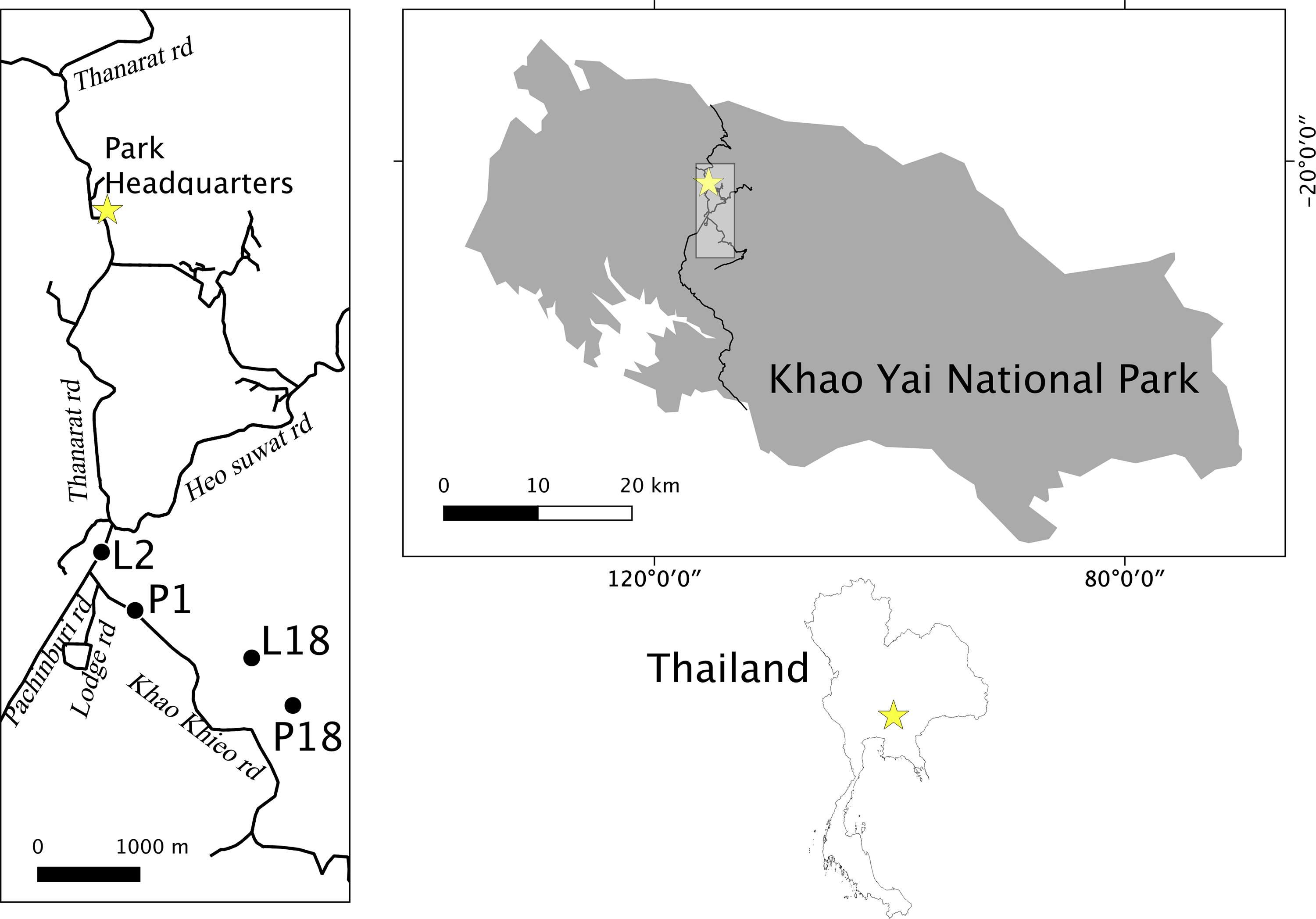
Figure 1. Location of roadside groups (L2, P1) and interior groups (P18, and L18) in Khao Yai National Park, Thailand.
Traffic volume
The park registered nearly a total of half a million vehicles during 2016 according to the National Park Wildlife and Plants Conservation Department, the national park with most traffic recorded in Thailand (DNP 2016). Nevertheless, to have an independent measure of traffic volume, we also counted the number of vehicles passing through the five roads (Thanarat road, Prachinburi road, Khao Khieo road, Heo Suwat, and Lodge road, Figure 1) that were located close to gibbon study groups. Vehicle counts were made from 8 to 11 am and from 1 to 4 pm on Wednesdays and Sundays during 8 months, and the mean daily number of vehicles was estimated for each one. We classified road volume levels as low (<100 mean number of vehicles a d-1: Khao Kiew and Lodge roads), medium (101–200: Hew Sawat road), and high traffic volume (>200: Thanarat and Prachinburi roads).
Gibbon study groups
We collected data on four habituated gibbon groups (two roadside groups: a Hylobates lar group, L2, and an H. pileatus group, P1, and two interior groups of each species, L18 and P18, living relatively near to roads, Figure 1) between March 2014 and November 2016 at the Klong Sai area of Khao Yai National Park. We followed groups from night tree to night tree, or for as long as possible in a given day, for a total 578 hr during non-consecutive 90 days (L18: 15 d, P18: 16 d, P1: 34 d, L2: 24 d).
Home-range and daily routes
We used a global positioning unit (GPS) to record the geographical locations of the focal group every minute in the Universal Transverse Mercator format (WGS84, Zone 47N). To estimate home ranges and delineate daily routes, we used only locations separated by intervals of 10 m. We calculated the minimum convex polygon (MCP) for all locations, which allowed setting the potential moving arena for later analyses on road band avoidance and movement simulations. To delineate home ranges, we used characteristic hull polygons, as they theoretically capture the potential effect of linear barriers on the final boundary shape (Downs & Horner Reference Downs and Horner2009; Getz et al. Reference Getz, Fortmann-Roe, Cross, Lyons, Ryan and Wilmers2007; José-Domínguez et al. Reference José-Domínguez, Savini and Asensio2015). This method uses a set of locations to create Delaunay triangles within the MCP. Then, area use is deduced from triangles’ perimeter: a small perimeter indicates high area use, while a large perimeter indicates low area use. Finally, to build the Delaunay home range, we plotted the sum of those triangles with a perimeter below two standard deviations. To delineate daily paths, we connected the 10-m interval locations in a map during each day in a continuous path line, which represented the gibbon daily route. We only mapped routes for days in which we observed gibbons during 5 or more hours.
Road crossing and predictor variables
Along with geographical coordinates, we recorded four other measures (i.e. four predictor variables) that described crossing locations: canopy gap, forest cover, forest length, and forest quality. Canopy gap was the minimum distance in meters between the branches on either side of the road that a gibbon used to cross over. To estimate forest cover, we used a spherical densiometer that averaged the percentage of points from the crossing location that did not enclose the sky across the four cardinal directions (Jennings et al. Reference Jennings, Brown and Sheil1999; Lemmon Reference Lemmon1956). Forest length was the percentage of forest that connected both sides of the road along a 100-m longitudinal road section, the crossing location being exactly at the centre of such a section. This is, the forest length was the percentage of forest that covered a road segment of 100 m, i.e. it was the “bridge width” of the crossing location. To have an estimate a forest quality, we first recorded the predominant forest type (primary, secondary, and no forest) at each side of the road considering a road segment of 100-m length and a 25 m perpendicular to both roadsides. We defined primary forest as that equivalent to mature forest with large trees therein, secondary forest corresponded to the successional stage or to damaged mature forest, and ‘no forest’ corresponded to grasslands and/or urban areas such as roads or buildings. Then, we used a five-point scale that accounted for quality considering both roadside forest types: ‘no forest-no forest’ (0), ‘no forest-secondary forest’ (1), ‘no forest-primary forest’ (2), and ‘secondary forest–secondary forest’ (3), ‘secondary forest–primary forest’ (4), ‘primary forest–primary forest’ (5). We also estimated the four measures for locations of the home range where gibbons did not cross the road with the constraint of a minimum distance of 100 m from any crossing or another non-crossing location to another crossing. In addition, we included other known crossing and non-crossing locations identified for other gibbon groups’ home ranges based on previous information available for Mo Sing To area, near the park headquarters (Asensio et al. Reference Asensio, Brockelman, Malaivijitnond and Reichard2011; Reichard et al. Reference Reichard, Ganpanakngan, Barelli, Kappeler and Watts2012; Savini et al. Reference Savini, Boesch and Reichard2008), pers. comm. by Warren Brockelman, and ad libitum observations. We calculated the four measures for each of the eight hundred thirty one 100-m length segments of the entire park road network.
Road avoidance
To estimate whether gibbons avoided roads, we first buffered roads in parallel bands of 25 m width within the MCP of each group (Figure S1). Then, we counted the number of locations in each distance band and estimated the expected frequency under an ideal free distribution: i.e. the proportion of band area multiplied by the total number of locations. The band avoidance index (cf. Laurance et al. Reference Laurance, Stouffer and Laurance2004)
illustrates avoidance or attraction to the related road band with positive values indicating avoidance to the corresponding band and negative values representing attraction to it.
To have further understanding of road avoidance, we investigated whether gibbons would have moved differently without any behavioural response to roads. To that aim, we compared observed gibbon movement against a null model that simulated movement using correlated random walks (Brehme et al. Reference Brehme, Tracey, McClenaghan and Fisher2013; Kareiva & Shigesada Reference Kareiva and Shigesada1983; Sheppard et al. Reference Shepard, Kuhns, Dreslik and Phillips2008; Whittington et al. Reference Whittington, Clair and Mercer2004). A correlated random walk assumes that the trajectory of an animal follows solely two parameters: the distribution of distances between successive locations and the distribution of turning angles between successive locations. We simulated 999 routes for each group (Figure S2) constrained within the MCP using the ‘adehabitat LT’ library in R (Calenge Reference Calenge2006). To have extra control on potential road avoidance, we also took as reference natural linear features of interior groups that should not virtually be a barrier to gibbon movement: a stream and elephant trail for L18 and P18 groups, respectively, and made the same comparison of observed versus simulated crossing rates over them.
Data analyses
We performed spatial analyses using QGIS (QGIS Development Team 2020) and statistical analyses using R (R Core Team 2020). The number of crossed and non-crossed locations was compared across traffic volume levels (low, medium, and high) using a Chi-Square Test (χ2) of independence. We compared the canopy gap, forest cover, forest length, and forest quality between crossed and non-crossed locations using Wilcoxon Mann–Whitney test. A log-likelihood ratio goodness-of-fit test (G-test), with Williams’s correction for sample size (Sokal & Rohlf Reference Sokal and Rohlf1995), compared whether there were differences in the number of locations in each road distance band versus expected frequencies. For each daily route, we calculated the road crossing rate: the number of times gibbons crossed roads km-1 travelled. Observed crossing rates were compared against simulated rates using Wilcoxon Mann–Whitney tests. To further control the effect of roads on gibbons’ travel paths, we also analyzed the crossing rate over linear natural features of interior groups’ home ranges: a stream and an elephant trail of less than 2 m width in L18 and P18 groups, respectively. Contrary to roads, these features were not expected to have any effect on the movement of gibbons, thus they added control to potential differences in observed crossing rates versus simulated crossing rates in roadside groups.
To assess the factors affecting the likelihood of gibbons crossing the road, we used GLMs with a logit link function, where crossing was the binary outcome variable (yes or no crossed at that location) and canopy gap, forest cover, forest length, and forest quality were the explanatory variables. We standardised explanatory variables by subtracting the mean from each value and then dividing it by its standard deviation. As the four variables were correlated with each other, we did not enter any of the predictor combinations together in the same model to keep independence among them. Thus, we ran four possible models to explain crossing probability, each of them containing a single predictor. For each model, we calculated the Akaike’s information criterion corrected for small samples (AICc) and obtained Akaike weights. Then, we averaged the set of models for which the cumulative weight was 0.95 (i.e. 95% probability of containing the best model, Burnham & Anderson Reference Burnham and Anderson2002) with the ‘model.avg()’ function of the MuMIn library in R, and obtained a mean estimate for each predictor (Bartón Reference Bartón2014). Finally, we used ‘predict()’ with the argument type ‘response’ to project the probability of crossing for each of the eight hundred thirty one 100-m segments of the park road network.
Results
Roads and home ranges
Roads traversed gibbons’ MCPs for a total length of 1639 m and 709 m in L2 and P1 groups, respectively (Figure 2). Home ranges of these groups were fragmented in various regions by roads, which were only connected by a few crossings: three regions and four crossings in the case of group L2, and two regions and five crossings in the case of group P1. Roads concurred with part of the edge of L2 home range, virtually making a barrier for part of it, though this was not the case of P1. These home range characteristics were not apparent in the interior groups, where natural linear features (the stream and the elephant trail) did not outline the home ranges of L18 and P18 groups.
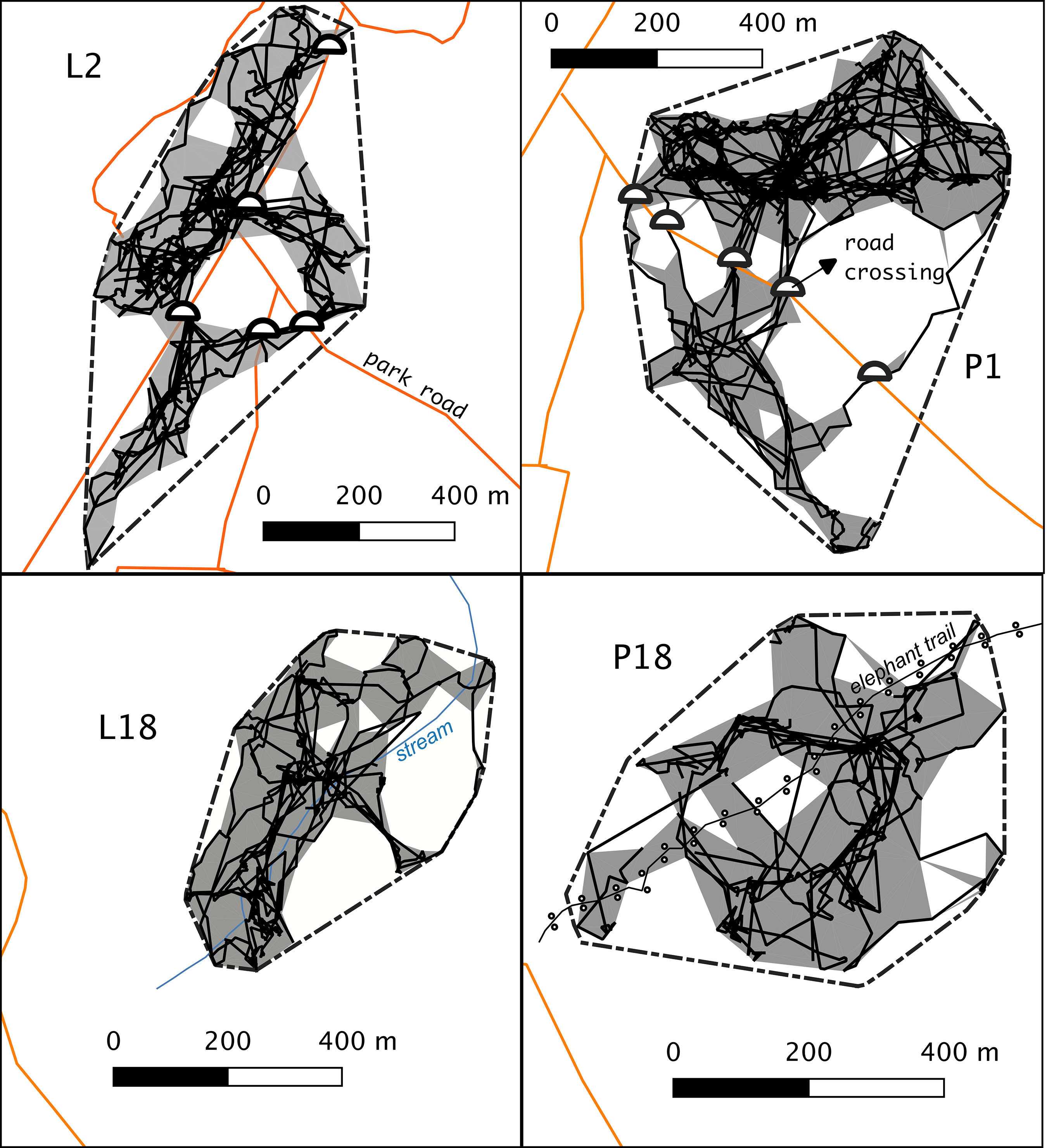
Figure 2. The road crossing locations (semicircles), home ranges (grey areas), home ranges (grey areas) and daily paths (black solid lines) of study gibbon groups. The outer polygon with dotted lines represents the minimum convex polygon (MCP) of all locations.
Crossing locations
We recorded 15 crossing locations. Nine crossing locations were made by L2 and P1 study groups (they shared one crossing), three more crossings were obtained from groups in Mo Sing To Area, around park headquarters, and another three were collected ad libitum at other road locations of the park. We also registered 15 known non-crossing locations within the MCP of followed road groups and within that of Mo SingTo area. There was no relationship between traffic volume and the location being crossed or not (χ2 = 3.41; df = 2; P = 0.18). Canopy gap, forest cover, forest length, and forest quality differed significantly between crossed and not crossed locations, with smaller canopy gaps at crossings compared to non-crossed locations (W = 309; P < 0.001); and greater forest cover (W = 48; P < 0.001); forest length (W = 96; P < 0.01); and forest quality scores (W = 85.5; P < 0.01; Figure S3).
Road avoidance
Gibbon groups showed significant differences in road band avoidance index (L2: G-test = 204.5, df = 9, P < 0.001; P1: G-test = 708.8, df = 20, P < 0.001; L18: G-test = 267.3, df = 24, P < 0.001; P18: G-test = 205.5, df = 30, P < 0.001; Figure 3). Roadside groups (L2 and P1) showed avoidance at the closest bands to roads, a pattern not present in interior groups (L18 and P18). However, such avoidance by roadside groups was not consistent as there was an irregular use of space as bands distanced from roads with bands that scored both avoidance and attraction, and some distant bands from roads presented avoidance scores.
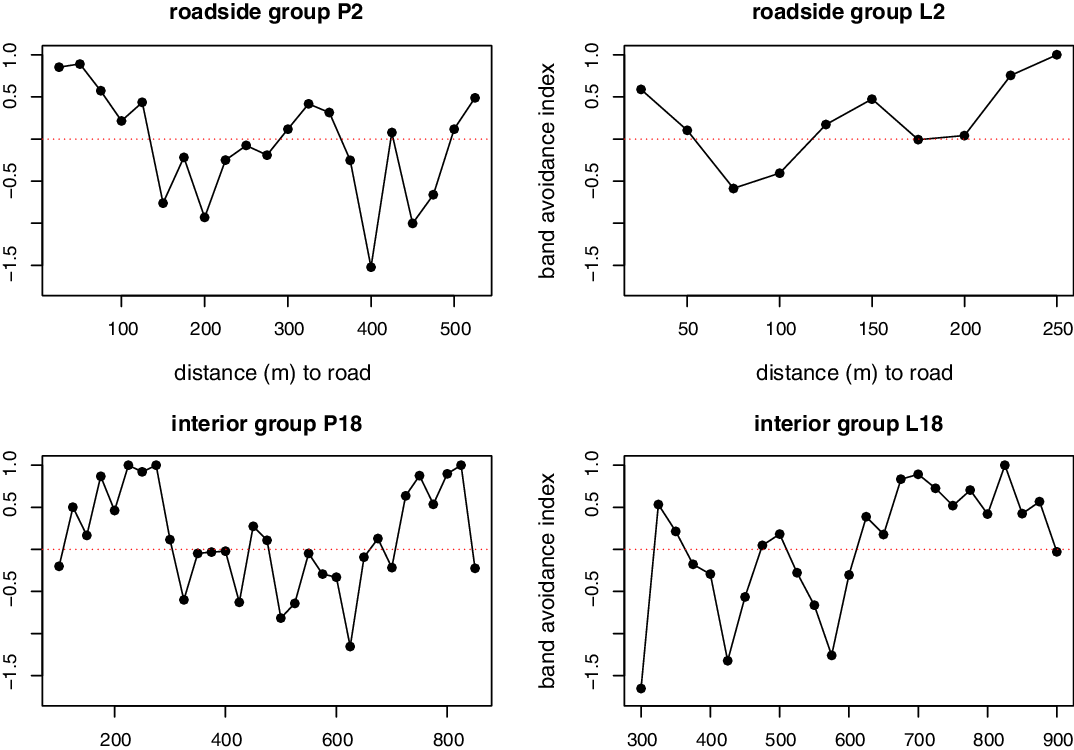
Figure 3. The band avoidance index of lar and pileated gibbons according to proximity to roads. Values above 0 (horizontal dotted line) represent avoidance to the corresponding road band distance and values below 0 attraction to it.
Pileated gibbons (P1) crossed roads at a mean rate (±SD) of 0.26 ± 0.56 times km−1, a rate smaller than that derived from simulated movement (mean = 1.32 ± 7.18; W = 22443; P < 0.05). Lar gibbons (L2) crossed roads nearly once per kilometer (mean = 0.93 ± 1.09), which was smaller compared to expected in simulated movement (mean = 3.20 ± 2.87; W = 6100, P < 0.001). Conversely, group P18 crossed the elephant trail at a rate of 1.36 ± 0.91 km−1, which was not significantly different than that obtained in simulations (mean = 1.96 ± 2.13; W = 12986, P = 0.68). Similarly, group L18 crossed the stream at a rate of 1.76 ± 1.94 km−1, which was not significantly different from that obtained in simulated movements (mean = 1.62 ± 2.19; W = 8132, P = 0.48).
GLM and park road network modelling
Two candidate GLM models comprised 95% of the Akaike weights explaining road crossings (Table S4). The averaged parameters of the two models revealed that both canopy gap and forest cover affected significantly the probability of gibbons crossing the road (Table 1): the larger the canopy gap the less probability of crossing (Figure 4a), and the greater the forest cover the more the probability of crossing (Figure 4b).
Table 1. Averaged parameter estimates of the best two models (according to the 95% confidence set of candidate models) affecting road crossing probability

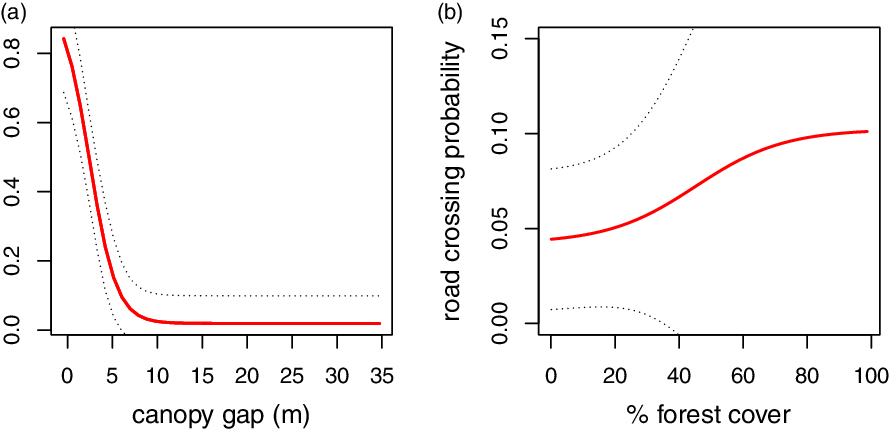
Figure 4. Relationship between predicted crossing probability (solid line) and canopy gap (a), and percentage of forest cover (b). Dashed lines represent the 95% confidence interval.
The averaged GLMs, considering canopy gap and forest cover in the 831 road segments, projected a mean (±SD) crossing probability of 0.35 ± 0.45 for the park road network (Figure 5). This prediction was highly bimodal with a peak of the road network particularly gathered at low crossing probabilities (close to 0), another one that peaked at high crossing probabilities (close to 1), and few road segments that obtained a middle probability. An important section of low crossing probability engrossed road sections around the headquarters and some touristic areas in the park (Figure 5b).

Figure 5. Road crossing probability for gibbons for the roads of Khao Yai National Park (a) and around park headquarters (b). the area around the park headquarters (b). Probability density function of the predicted crossing probability (c), vertical dashed line indicates the mean.
Discussion
Managers of protected areas have the duty of mitigating the unavoidable impact of roads on resident fauna and flora. Roads fragmented gibbons’ home ranges of roadside groups in a few regions, particularly in that of L2 group, which contained a relatively long road therein. Nevertheless, park roads were not an impenetrable ‘fortification’ to movement, and gibbons traversed them at a few locations. In some occasions, solitary gibbons crossed the road by the ground to escape from the aggression of resident individuals (pers. obs.). This means that in certain extreme situations, such as avoiding territorial aggression, gibbons will not hesitate to use the road substrate, with all the risk that this would entail regarding vehicle collision or predation. Thus, although roads were not indeed absolute barriers, our results suggest that they create canopy gaps and low forest coverage, which determine gibbon movement across the park landscape.
Roadside gibbons had some reluctance to being nearby roads, but we could not find a clear pattern of space use decline as they approached them. For example, some incoherent road avoidance by gibbon groups appeared in the most distant home-range bands, and interior groups did not have avoidance scores in the closest bands. This is likely the product of factors other than proximity to roads explaining avoidance or attraction to a particular distance band. Animals might emphasise their ranging effort in particularly food-productive regions of the home range (Asensio et al. Reference Asensio, Brockelman, Malaivijitnond and Reichard2014); avoid staying at its edges because of overlap with neighbours where conflicts might arise (Reichard & Sommer Reference Reichard and Sommer1997; Suwanvecho & Brockelman Reference Suwanvecho and Brockelman2012); or simply, tend to use the centre of the range to reduce the costs of returning to a preferred centre from its edges (Wranghan et al. Reference Wrangham, Crofoot, Lundy and Gilby2007). Nonetheless, observed road crossing rates were smaller than those generated by correlated random walks, which represented a null model where roads would not affect gibbon movement. This difference indicates that roads constrained the movement of gibbons, as they did not move freely across the potential moving arena. Other natural linear features (a stream and an elephant trail) within the ranges of interior groups did not differ in how often gibbons crossed them compared to simulations, further supporting that the effect of roads on the movement of gibbons was not fortuitous at roadside groups. This reluctance suggests that gibbons approached roads to move between areas of foraging, resting, or social activities, but did not use roadside areas themselves intensively.
The best model explaining crossing probability included forest cover, with a positive effect, and canopy gap, with a particularly strong negative effect. This corroborates that a width canopy opening is a primary factor restricting road crossings in arboreal taxa (Asari et al. Reference Asari, Johnson, Parsons and Lason2010, van der Ree et al. Reference van der Ree, Cesarini, Sunnucks, Moore and Taylor2010). Asensio and colleagues (Reference Asensio, Murillo-Chacon, Schaffner and Aureli2017) suggest that the spider monkey, a highly arboreal primate species, deals with the canopy gap created by roads better than other arboreal taxa due to their flexible arboreal locomotion. We believe that this is also the case for gibbons confronting roads as both gibbons and spider monkeys are remarkable at using suspensory locomotion to make fluid transitions through the canopy (Cheyne Reference Cheyne, D’Août and Vereecke2011; Robbins et al. Reference Robbins, Chapman and Wrangham1991). Gibbons may logically feel more willing to cross the road if not only the necessary branch distance is reachable, but also if forest coverage makes the alien road substrate less evident compared to familiar forested habitat. Even some understory bird species, avoid roads and the edge-affected habitat near it (Laurance et al. Reference Laurance, Stouffer and Laurance2004). Similarly, in a different study, birds flew away from roads if vegetation was lower on the opposite side of the road than the side where the bird was sitting (Husby & Husby Reference Husby and Husby2014). Spider monkeys also did not pass over roads at locations with high habitat disparity between roadsides (Asensio et al. Reference Asensio, Murillo-Chacon, Schaffner and Aureli2017). Thus, gibbons may tolerate relatively well the presence of roads in their territories if the damage made to the roadside vegetation of particular bridging locations is minimum.
We found a bimodal crossing probability for gibbons when making predictions for the entire park road network. That is, although crossing probability peaked at several road segments, there was also an important part of segments approaching zero probability. This polarity in crossing probability makes it easy to identify the road areas for park managers that are already safe for arboreal taxa and those that require specific attention. Low probabilities were particularly evident around headquarters, where most anthropogenic facilities of the park are set (touristic areas, restaurants, souvenir shops, camping sites…). In fact, three out of six known road groups in this area of the park never crossed the road at any location, and their home ranges did not contain roads despite being next to them (Asensio et al. Reference Asensio, Brockelman, Malaivijitnond and Reichard2011; Reichard et al. Reference Reichard, Ganpanakngan, Barelli, Kappeler and Watts2012; Savini et al. Reference Savini, Boesch and Reichard2008). It would be interesting to investigate whether there is some genetic differentiation of populations on opposite sides of long sections of non-crossed road segments as observed elsewhere (Gerlach & Musolf Reference Gerlach and Musolf2000; Keller & Largiadèr Reference Keller and Largiadèr2003; Reh & Seitz Reference Reh and Seitz1990). Part of the road network traverses a contact zone where both pileated and lar gibbons interact and mixed and hybrid groups also exist (Brockelman & Gittins, Reference Brockelman, Gittins, Preuschoft, Chivers, Brockelman and Creel1984; Marshall et al. Reference Marshall, Ross and Chantharojvong1972), and thus, roads could compromise the unique interspecific dynamics of this zone by intensifying the potential barrier between the two species.
Despite a high proportion of road segments having low crossing probabilities, the mean crossing probability for the park network was 0.33. Thus, the relatively short and narrow road network at the site might be within the standards of how roads should be built in a protected area. Besides, the road network only crosses a small portion of the park and thus many regions, particularly those to the east, remain safe from any impact of roads (Figure 1). Nevertheless, we need to ponder that these road arrangements were not made explicitly to minimise road damage, but were rather the consequence of Khao Yai National Park being placed in a mountainous region with difficulties for easier road development (Ruhle Reference Ruhle1964). Low-elevation forested areas usually suffer a higher level of anthropogenic disturbance compared to steep high-elevation forests (Holmes Reference Holmes, Wikramanayake, Dinerstein and Loucks2002). Therefore, most protected areas in Thailand are set in mountainous areas probably because lowland areas were either converted into agricultural fields or urban areas much earlier than the settlement of protected areas (Turner & Corlett Reference Turner and Corlett1996). The nearby highway that divides Khao Yai and Tap Lan National Parks is a suitable example of this observation as it consists of a relatively flat area separating the two mountainous park areas. The highway is composed of sections of up to five-lane roads and has a heavy traffic volume all year round. This highway constitutes a ‘deadly barricade’ to animals that attempt traversing it with an estimate of 9684 vertebrate kills by vehicle collision per year (Silva et al. Reference Silva, Crane and Savini2020). This linear infrastructure virtually isolates the population of the pileated gibbon into Khao Yai Park by discontinuing dispersal from its main geographical distribution to the east (Asensio et al. Reference Asensio, Murillo-Chacon, Schaffner and Aureli2017).
We did not find a relationship between the existence of a crossings and the traffic level at such a road. This does not mean that gibbons are not sensible to vehicles passing when they have to pass over roads. Since the analysis focussed on the somewhat permanent (at least during the study time) characteristics of the crossing locations, it could not capture whether gibbons avoided cars when making crossing decisions. Besides, the same road contained crossing and not crossing locations with logically the same traffic level, which hindered understanding the vehicle effect on crossing behaviour. Therefore, it would be important to understand if gibbons cross roads at periods when no cars or less traffic occurs. This would also allow park managers to regulate speed limit and vehicle levels during particular dates and hour frames to facilitate gibbon movement at known crossings.
An obvious recommendation to increase the mobility of arboreal taxa in protected areas is to let grow or improve roadside vegetation in those areas with low crossing opportunities such as the park headquarters’ whereabouts. Management plans should focus on improving the damaged forest canopy by roads, or at the very least not cut the trees and branches of known crossing locations. Considering the high territoriality displayed by gibbon groups (Raemeakers & Raemeakers Reference Raemeakers and Raemeakers1985), dispersing individuals may have difficulties to cross roads using the locations that belong to other roadside groups’ home ranges. During the last weeks of this study, the park staff set a canopy bridge at one of the crossing locations of group L2 to ease their crossing, and gibbons used it (Saralamba, pers. comm.). Thus, we advocate setting more artificial bridges linking canopies at about 1 km intervals for those long sections of the road with low crossing probability. Building more artificial bridges at key locations would increase the functional landscape connectivity (Taylor et al. Reference Taylor, Fahrig, Henein and Merriam1993) of gibbons and also of other arboreal taxa at the site. Future research should focus on monitoring, censusing, and assessing the status of known crossing locations by also accounting for how often and when they are used by arboreal animals.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0266467421000390
Acknowledgements
We acknowledge the Department of National Parks (DNP) and the National Research Council (NRCT) of Thailand for providing permission to conduct this research. We want to thank Warren Brockelman, Tommaso Savini and Eduardo Ogando for advice and insights on this study.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interests
None.
Ethical standards
This research is solely observational and adheres to the legal requirements of Thailand with the permit id 0907.4/17635 provided by the Department of National Parks, Wildlife and Plant Conservation.











