INTRODUCTION
Sirenians are known to consume large quantities of vegetation (Bengtson, Reference Bengtson1981, Reference Bengtson1983; Best, Reference Best1981; Marsh et al., Reference Marsh, Channells, Heinsohn and Morrissey1982; Hurst & Beck, Reference Hurst and Beck1988). The large-scale grazing habit of these marine mammals is thought to have a positive impact on species biodiversity in seagrass beds (Packard, Reference Packard1984) and a positive effect on the structure and dynamics of these communities (Aragones & Marsh, Reference Aragones and Marsh2000). Knowledge of the diet requirements of sirenians is important for protecting the habitats in which these endangered species reside.
The Antillean manatee (Trichechus manatus manatus), a subspecies of the West Indian manatee (Trichechus manatus), occupies shallow coastal waters surrounding many islands throughout the central Caribbean and also shallow waters adjacent to mainland areas from the Yucatan Peninsula of Mexico south to Alagoas State, Brazil. Manatee numbers within this range are relatively low despite their widespread distribution and suitable habitat to support a larger population (Rathbun et al., Reference Rathbun, Powell and Cruz1983; Reynolds et al., Reference Reynolds, Szelistowski and Leon1995; Perez, Reference Perez2005). There is, however, an estimated population of 1000 manatees within Belize (Auil Gomez, Reference Auil Gomez, Palomares and Pauly2011), and the coastal waters adjacent to Belize are recognized as a critical habitat for the subspecies' continued survival (Quintana-Rizzo & Reynolds, Reference Quintana-Rizzo and Reynolds2007). A Manatee Recovery Plan has been enacted by the Belizean government to protect the manatee population (Auil, Reference Auil1998), but the most recent population trends suggest a decline in what was previously thought to be a stable population (Auil, Reference Auil2004). Because of declining numbers and low genetic variation in Antillean manatee populations (Hunter et al., Reference Hunter, Mignucci-Giannoni, Pause Tucker, King, Bonde, Gray and McGuire2012), conservation of these manatees is a priority.
Belize contains favourable manatee habitat: 386 km of coastline along with several rivers, tidal lagoons, barrier islands (cayes) to the east, and mangrove lagoons bordering the Mesoamerican Barrier Reef. Recent surveys indicated that manatees were most numerous in the region from Chetumal Bay in the north to Placencia Lagoon in the south and east to the cayes off Belize City (Morales-Vela et al., Reference Morales-Vela, Olivera-Gomez, Reynolds and Rathbun2000; Auil, Reference Auil2004; Edwards et al., Reference Edwards, Stone, Hines, Auil Gomez and Winning2014). These areas are lined with seagrass beds extending from the shoreline to the outer-lying cayes and islands, a coastal system of lagoons and bays that connect with the Caribbean (CZMAI, 2014), as well as networks of freshwater rivers that provide manatees with access to drinking water sites.
Numerous studies have focused on the types of food that sirenians consume in the wild (Best, Reference Best1981; Marsh et al., Reference Marsh, Channells, Heinsohn and Morrissey1982; Gallivan & Best, Reference Gallivan and Best1986; Mignucci-Giannoni & Beck, Reference Mignucci-Giannoni and Beck1998; Lanyon & Sanson, Reference Lanyon and Sanson2006; Castelblanco-Martinez et al., Reference Castelblanco-Martinez, Morales-Vela, Hernandez-Arana and Padilla-Saldivar2009). Several plant species have been documented as occurring within the digestive tract of Florida manatees (T. manatus latirostris) (Best, Reference Best1981; Ledder, Reference Ledder1986; Hurst & Beck, Reference Hurst and Beck1988), Antillean manatees (Mignucci-Giannoni & Beck, Reference Mignucci-Giannoni and Beck1998; Castelblanco-Martinez et al., Reference Castelblanco-Martinez, Morales-Vela, Hernandez-Arana and Padilla-Saldivar2009; Navarro-Martinez et al., Reference Navarro-Martinez, Alvarez-Aleman and Castelblanco-Martinez2014), Amazonian manatees (T. inunguis) (Colares & Colares, Reference Colares and Colares2002; Guterres-Pazin et al., Reference Guterres-Pazin, Marmontel, Rosas, Pazin and Venticinque2014) and the dugong (Dugong dugon) (Heinsohn & Birch, Reference Heinsohn and Birch1972; Lipkin, Reference Lipkin1976; Marsh et al., Reference Marsh, Channells, Heinsohn and Morrissey1982). Best (Reference Best1981) conducted the first comprehensive review on the feeding behaviour, digestive physiology, consumption and diet of sirenians in wild and captive settings. A study focused on the diet of the Amazonian manatee identified a total of 24 different macrophytes from digestive tract and faecal samples with higher diversity in types of vegetation consumed during the dry season; manatees appeared to be more selective on what they consumed during the wet season (Colares & Colares, Reference Colares and Colares2002; Guterres-Pazin et al., Reference Guterres-Pazin, Marmontel, Rosas, Pazin and Venticinque2014). Some of the most comprehensive diet data for manatees are specific to the Florida manatees (Bengtson, Reference Bengtson1983; Hurst & Beck, Reference Hurst and Beck1988; Ames et al., Reference Ames, Van Vleet and Sackett1996; Alves-Stanley et al., Reference Alves-Stanley, Worthy and Bonde2010). In Florida, manatees are known to consume over 60 different species of plants (Hartman, Reference Hartman1979; Bengtson, Reference Bengtson1981, Reference Bengtson1983; Best, Reference Best1981; Hurst & Beck, Reference Hurst and Beck1988) of varying nutritional value (Siegal-Willott et al., Reference Siegal-Willott, Harr, Hayek, Scott, Gerlach, Sirois, Reuter, Crewz and Hill2010). Antillean manatees in Puerto Rico were reported to consume 10 different species of vegetation (Mignucci-Giannoni & Beck, Reference Mignucci-Giannoni and Beck1998). A wide range of estimates exists with regard to the amount of food manatees consume in a given day, from 9 to 80 kg (Severin, Reference Severin1955; Crandall, Reference Crandall1964; Pinto de Silveira, Reference Pinto da Silveria1975; Hartman, Reference Hartman1979; Best, Reference Best1981; Lomolino & Ewel, Reference Lomolino and Ewel1984), and between 4–9% (Bengtson, Reference Bengtson1983) to 10–15% of their body weight per day (Reep & Bonde, Reference Reep and Bonde2006). The quantity of vegetation eaten is dependent on the animals' size, activity level, nutritional value of the food source, metabolic requirements and perhaps reproductive condition. Consumption is also influenced by the availability of plants, which is dependent on a broad suite of environmental conditions.
Manatees select habitat based on the availability of food and proximity to freshwater resources (Hartman, Reference Hartman1979; Packard & Wetterqvist, Reference Packard and Wetterqvist1986; O'Shea & Kochman, Reference O'Shea, Kochman, Reynolds and Haddad1990; Gannon et al., Reference Gannon, Scolardi, Reynolds, Koelsch and Kessenich2007). In order to meet their metabolic requirements, manatees must consume large quantities of vegetation on a daily basis (Smith, Reference Smith1993). Seagrasses are an important food source for West Indian manatees and unfortunately, there have been documented declines in seagrass productivity (biomass) and biodiversity in many areas (Short & Wyllie-Echeverria, Reference Short and Wyllie-Echeverria1996; Duarte, Reference Duarte2002; Orth et al., Reference Orth, Carruthers, Dennison, Duarte, Fourquarean, Heck, Hughes, Kendrick, Kenworthy, Olyarnik, Short, Waycott and Williams2006; Waycott et al., Reference Waycott, Duarte, Carruthers, Orth, Dennison, Olyarnik, Calladine, Fourquarean, Heck, Hughes, Kendrick, Kenworthy, Short and Williams2009), including Belize (Short et al., Reference Short, Koch, Creed, Magalhaes, Fernandez and Gaeckle2006; Parham-Garbutt, Reference Parham-Garbutt2015). Manatees also face habitat loss within many parts of their range which limits the resources necessary for their survival (Smith, Reference Smith1993).
Several methods have been used to study the diet of herbivores, including direct observation and examination of ingesta and faecal samples using microhistological analysis employing a microscope point technique (Hurst & Beck, Reference Hurst and Beck1988). Microhistological analysis is a favoured method for the identification of ingesta and faecal samples collected from terrestrial herbivores (Holechek & Vavra, Reference Holechek and Vavra1981) and has been employed also to study the diets of aquatic herbivores (Owen, Reference Owen1975; Black et al., Reference Black, Prop, Hunter, Woog, Marshall and Bowler1994; Carriere et al., Reference Carriere, Bromley and Gauthier1999; Castelblanco-Martinez et al., Reference Castelblanco-Martinez, Morales-Vela, Hernandez-Arana and Padilla-Saldivar2009; Flores-Cascante et al., Reference Flores-Cascante, Morales-Vela, Castelblanco-Martinez, Padilla-Saldivar and Auil2013) including sirenians (Channels & Morrissey, Reference Channels, Morrissey and Marsh1981; Hurst & Beck, Reference Hurst and Beck1988; Mignucci-Giannoni & Beck, Reference Mignucci-Giannoni and Beck1998). This modified microscope point technique has proved to be an effective, yet inexpensive, method to gather information on diets of herbivores, and the methodology outlined by Hurst & Beck (Reference Hurst and Beck1988) was followed for this study.
Here we provide the first in-depth information on manatee diet in Belize. Two high-use areas of Belize were compared to determine locational differences in manatee diet and to determine which habitats are likely to provide important resources for manatees. A better understanding of how Antillean manatees utilize these different areas will be necessary to develop and implement effect management plans for this endangered species.
MATERIALS AND METHODS
Subjects and sampling areas
Samples were obtained from manatees temporarily retained for telemetry studies at two locations in Belize (Figure 1): Southern Lagoon (17°12′40″N 88°20′17″W) near Gales Point, Belize District from 1997–2008, 2010 and 2012, and the Drowned Cayes (17°29′25″N 88°08′10″W) off Belize City, Belize District from 2004–2007, 2012 and 2013. Ingesta also were obtained at necropsy from manatee carcasses collected near Belize City in 1999, 2002, 2003, 2008 and 2013.
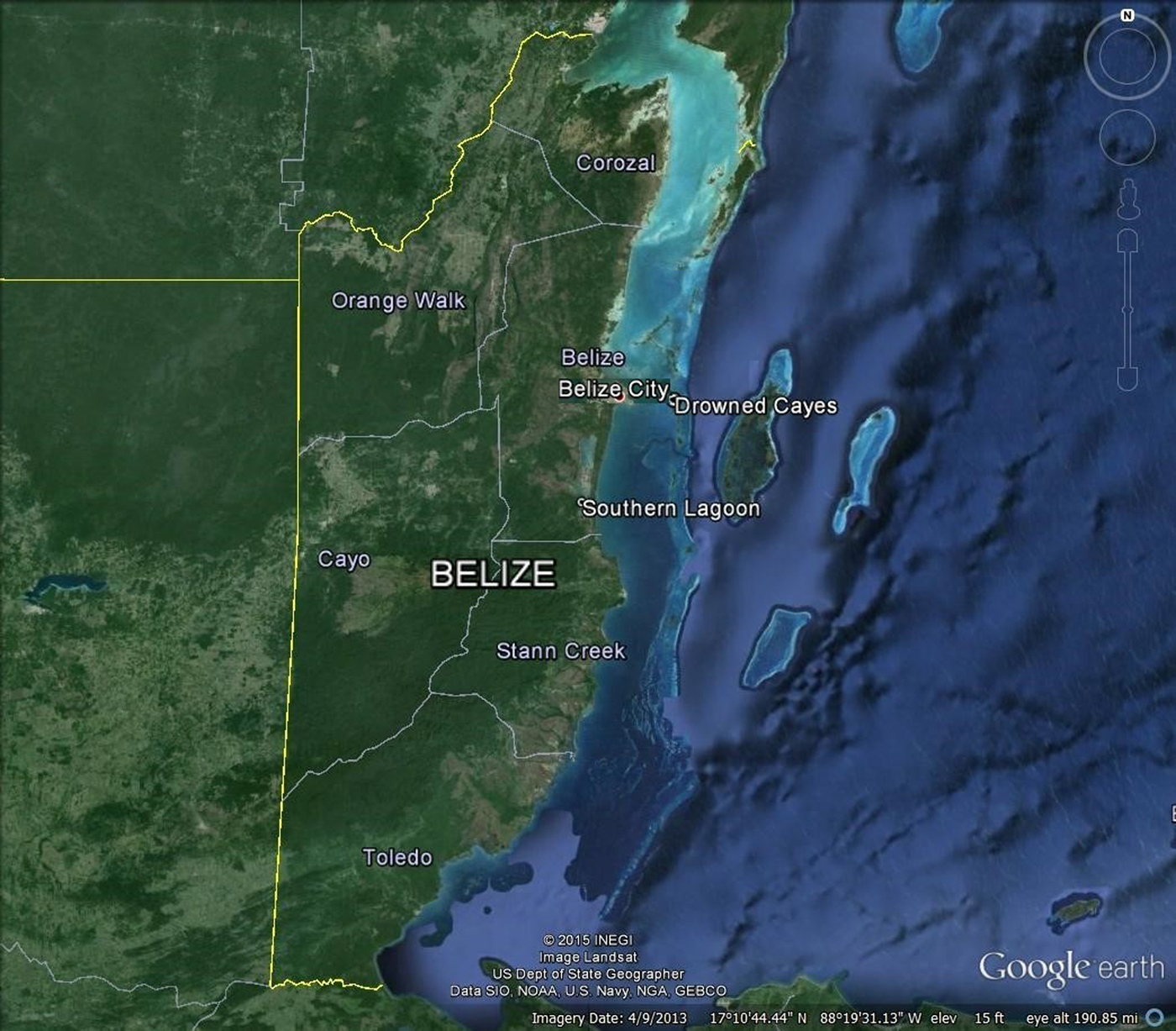
Fig. 1. District map of Belize showing areas of sample collection in the Drowned Cayes and Southern Lagoon, and carcasses collected near Belize City.
Collection methods
From 1997–2013, health assessments were conducted in Belize as part of a tagging study involving the capture and release of wild manatees. Individual manatees were net captured by an experienced crew from Florida and Belize, and pulled onto land or onto the deck of a specialized boat for a detailed physical examination (Bonde et al., Reference Bonde, Garrett, Berlanger, Askin, Tan and Wittnich2012; Wong et al., Reference Wong, Bonde, Siegal-Willott, Stamper, Colee, Powell, Reid, Deutsch and Harr2012). Routine measurements and biological samples were collected from 137 manatees, including 13 mouth and 124 faecal content. Additional ingesta samples from the stomach (4), duodenum (1) and colon (1) were obtained from six manatee carcasses (collected by the Belize Marine Mammal Stranding Network), yielding a total of 143 samples for analysis. In total, 111 samples were taken from animals captured in Southern Lagoon and 32 from animals captured in the Drowned Cayes. All samples were preserved in 70% EtOH upon collection and stored until ready for examination.
Microhistological analysis
Samples were not processed to achieve a uniform size as has been done in other diet studies, since a large fragment size was preferred to enable easier identification. Prior to examination, each sample was rinsed with tap water over a 30-mesh (0.52 mm) screen to remove sand, dirt and other fine particulate matter that might obscure microscopic observation of plant cellular structures. After rinsing, a subsample of the digesta was placed on a 2 × 3 inch glass slide to which several drops of Hertwig's solution was added. The slide was then held over an alcohol flame to facilitate the clearing of pigments from the plant cells, allowing for easier viewing of cellular structures. After clearing, the subsample was divided onto five additional 2 × 3 inch glass slides for microhistological examination.
During microscopic observation, each slide was first observed at 40× for the purpose of scanning the contents. Samples were then observed at 100× and analysed for content. Each slide was analysed through the microscope by identifying five points visible in an eyepiece micrometer grid along a prescribed transect sequence at 20 different coordinates on the stage; observations were recorded at each coordinate. This allowed for 100 different points of identification on each slide and was repeated five times for each sample. Using this modified microscope point technique developed by Owen (Reference Owen1975) and Holechek et al. (Reference Holechek, Gross, Dabo and Stephenson1982a, Reference Holechek, Vavra and Pieperb), and detailed in Hurst & Beck (Reference Hurst and Beck1988) and Beck & Clementz (Reference Beck, Clementz, Hines, Reynolds III, Aragones, Mignucci-Giannoni and Marmontel2012), 500 points in each sample were identified using microhistological characters of visible plant fragments. The frequency of items was identified for the overall diet of manatees in Belize by recording the number of times each item was found in a sample, divided by the total number of samples studied.
To assist with identification, plant fragments were compared with reference voucher slides and photomicrographs available at the USGS Sirenia Project laboratory, along with illustrations from Hurst & Beck (Reference Hurst and Beck1988) that describe leaf, stem, flower, root and rhizome fragments of over 100 plant species catalogued for the study of manatee diet through microhistological examination. Some species of algae were identified using field guides (Littler et al., Reference Littler, Littler, Bucher and Norris1989; Littler & Littler, Reference Littler and Littler2008) and outside expert analysis. Photomicrographs of some algal fragments and intact samples were sent to Dr Donald W. Ott at the University of Akron for identification by election microscopy. Photographs were obtained of some ingested items for subsequent confirmation of identification.
Data analysis
Samples from manatees in Belize were divided into groups and evaluated based on location, sex, size classification and season. Size classifications for manatees were based on records maintained for 280 manatee health assessments conducted in Belize, and defined as follows: calves (<200 cm), juveniles (>200–<245 cm) and adults (>245 cm). Per cent frequency of species observed was determined by adding the total count of each species type detected in the samples, divided by the total number of samples analysed for each group, and then divided by 500 (see Microhistological analysis), i.e. ![]() ${P_F = n_{sp} \over 500}$. Diet composition was also described through per cent occurrence of samples analysed across four categories: seagrasses, other vascular plant material, algae and invertebrates. Variation in per cent occurrence of seagrasses, other vascular plant material, and algae was further analysed statistically as a factorial analysis of variance (ANOVA) with main effects and interactions for locality, sex, size classification and season. Prior to analysis, the data were transformed to ranks to adjust for the non-normal distribution of percentages. All statistical analyses were carried out using the SAS PROC GLM utility (SAS Institute Inc., 2013).
${P_F = n_{sp} \over 500}$. Diet composition was also described through per cent occurrence of samples analysed across four categories: seagrasses, other vascular plant material, algae and invertebrates. Variation in per cent occurrence of seagrasses, other vascular plant material, and algae was further analysed statistically as a factorial analysis of variance (ANOVA) with main effects and interactions for locality, sex, size classification and season. Prior to analysis, the data were transformed to ranks to adjust for the non-normal distribution of percentages. All statistical analyses were carried out using the SAS PROC GLM utility (SAS Institute Inc., 2013).
RESULTS
Microhistological analysis revealed the contents of each sample by identifying plant fragments through microscopic investigation. The fragment sizes in mouth and stomach samples were larger than those found in faecal samples. Mouth samples contained almost complete, intact pieces of plant material, thus facilitating identification. As samples progressed further along the digestive tract, advancing from the stomach to distal colon, sample integrity and the ease of identifying fragments decreased due to digestive processes. When possible, ingesta were identified to genus as well as fragment component (e.g. leaf, stem, rhizome), then categories of species were summarized based on their occurrence within the samples.
Components of the manatee diet in Belize
The number of unique species identified in each sample ranged from 1 to 6. Mouth samples consisted of 1–2 species of seagrass, whereas 1–4 species were identified in GI-tract samples, and 1–6 different species were identified in faecal samples. Most samples, however, contained fragments from more than one diet category. Of the 143 samples, mixed seagrass rhizome was found in 129 (90.21%) samples. The seagrass Halodule wrightii was the second most frequently detected dietary item and occurred in 114 (79.72%) samples. In samples with mixed seagrass rhizome, the exact species of seagrass was not always discernible. Overall, 15 different items were observed in samples in addition to the highest ranked component of seagrass rhizome (Table 1). The relative occurrence of the dietary items in all samples (N = 143) by category is summarized in Table 2.
Table 1. Per cent frequency of items identified in manatee ingesta.
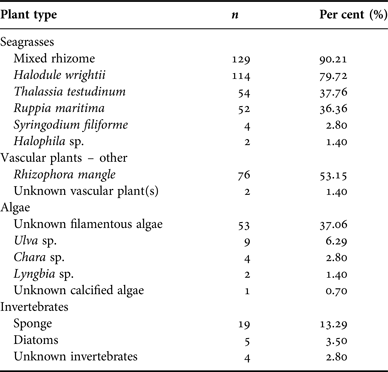
Table 2. Per cent occurrence of diet items in 143 Belize manatee samples, summarized by plant category.
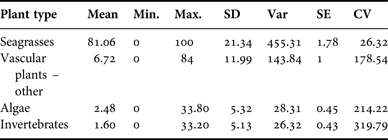
All 143 samples were examined to identify per cent occurrence relative to 500 identification points in each sample (Table 2) and to classify the average composite dietary make-up of each specimen. Identities were type categorized as ‘seagrasses’ (Halodule wrightii, Thalassia testudinum, Ruppia maritima, Syringodium filiforme, Halophila sp. and seagrass rhizome), ‘vascular plant(s) – other’ (e.g. Rhizophora mangle and unidentified vascular plant), ‘algae’ (e.g. Ulva sp., unidentified filamentous algae), or ‘invertebrates’ (e.g. unknown poriferan, unknown invertebrate). Seagrass leaf and/or rhizome had the highest average per cent occurrence in the diet (Table 2). In several samples, there were some fragments that were unidentifiable at the time of observation. Since it was not possible to classify these fragments, they were disregarded for analytical purposes.
Manatee diet by location
Diet contents were analysed from samples collected in two locations (Appendix A). Seagrass rhizome was the predominant ingested dietary item at both Southern Lagoon and the Drowned Cayes (N = 111, 90.09% frequency in Southern Lagoon; N = 32, 90.63% in the Drowned Cayes). Halodule wrightii was the most frequently identified component from both Southern Lagoon (N = 85, 76.58%) and the Drowned Cayes (N = 29, 90.63%). Rhizophora mangle and Ruppia maritima also were common in samples from Southern Lagoon, as were Thalassia testudinum and Rhizophora mangle in samples from the Drowned Cayes. Per cent frequency of other dietary components by location is provided in Appendix A.
The average per cent occurrence from samples obtained in Southern Lagoon and the Drowned Cayes indicated that seagrass was a primary component in specimens from Southern Lagoon: 83.14% (SD = 20.87) and the Drowned Cayes: 73.18% (SD = 21.89). Per cent occurrence to classify the composite dietary sample by location is provided in Appendix B. In a reduced three-factor ANOVA (to account for no significant difference by sex), locality had a significant effect on seagrass consumption (P = 0.03) due to a higher estimated mean in Southern Lagoon (83.5 ± 2.12) compared with the Drowned Cayes (70.5 ± 4.58). However, per cent consumption of both mangrove and algae were significantly higher (P = 0.03) in the Drowned Cayes (mangrove: 14.7 ± 2.35, algae 4.6 ± 1.00) when compared with Southern Lagoon (mangrove: 5.4 ± 1.08, algae 1.5 ± 0.46).
Manatee diet by sex
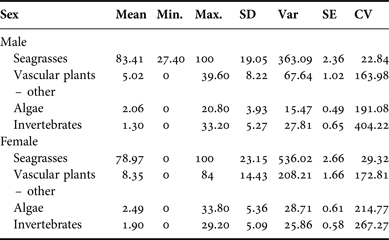
Samples were collected from 65 males and 76 females. Samples from both male and female manatees contained primarily seagrass rhizome and leaf material, with an average of 83.41% (SD = 19.05) seagrass in males and an average of 78.97% (SD = 23.15) seagrass in females. There was no significant difference in diet composition by sex (P > 0.09). Per cent frequency of other dietary components by sex is provided in Appendix C; per cent occurrence to classify the composite dietary sample by location is provided in Appendix D.
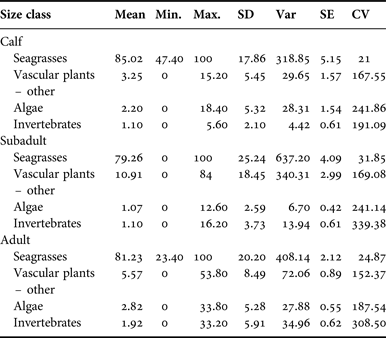
Manatee diet by size classification
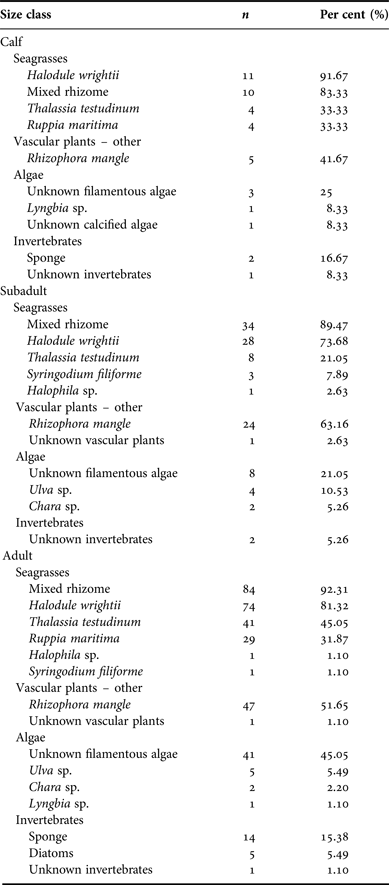
For all size classifications, diets were very similar with mixed seagrass rhizome, Halodule wrightii, and Rhizophora mangle (red mangrove) occurring most frequently in both locations. Per cent frequency of other dietary components compared between size classifications is provided in Appendix E.
Size classifications also were analysed to examine per cent occurrence between each plant type. Seagrasses were again the type seen most frequently. Samples from manatee calves contained a mean 85.02% seagrass (SD = 17.86), juvenile manatee samples averaged 79.26% seagrass (SD = 25.24), and adult samples consisted of a mean of 82.23% seagrass (SD = 20.20). Per cent occurrence to classify the composite dietary sample by location is provided in Appendix F. Per cent algae consumption was also significantly affected by size classification (P = 0.04) due to a higher estimated mean for manatees >245 cm (3.8 ± 0.65) compared with manatees ≤245 cm (2.3 ± 0.89).
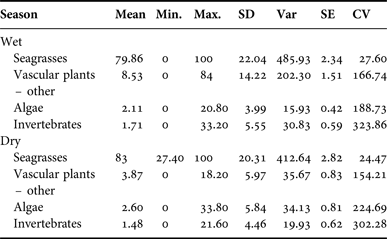
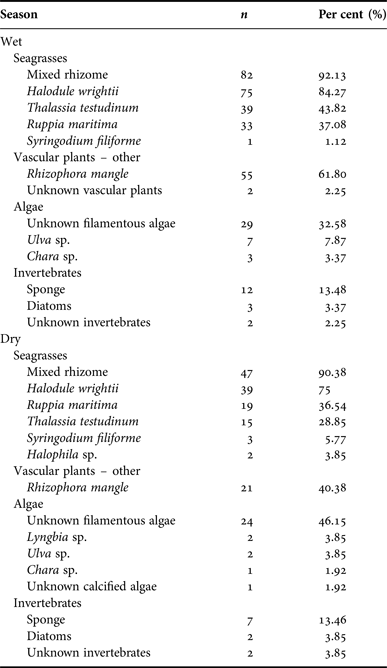
Manatee diet by season
Belize experiences a wet season from June to November, with peak rainfall in July and August, and a dry season from December to May. Samples from the wet (N = 89) and dry season (N = 52) contained rhizome in the majority of samples (wet = 82, 92.13%, dry = 47, 90.38%), followed by Halodule wrightii (wet = 75, 84.27%, dry = 39, 75.00%). Wet and dry seasons were compared by per cent occurrence of each type. Seagrass occurred most frequently in samples from both seasons. Wet season samples contained 79.86% seagrass, while samples from the dry season contained 83.00% seagrass (SD = 20.31). There was no significant difference in diet composition by season (P > 0.30). Per cent frequency of other dietary components by sex is provided in Appendix G; per cent occurrence to classify the composite dietary sample by location is provided in Appendix H.
DISCUSSION
The items present in diet samples from this study in Belize have been previously reported for manatees in other locations throughout the Atlantic and broader Caribbean regions (Best, Reference Best1981; Ledder, Reference Ledder1986; Hurst & Beck, Reference Hurst and Beck1988; Mignucci-Giannoni & Beck, Reference Mignucci-Giannoni and Beck1998; Borges et al., Reference Borges, Araujo, Anzolin and Miranda2008; Castelblanco-Martinez et al., Reference Castelblanco-Martinez, Morales-Vela, Hernandez-Arana and Padilla-Saldivar2009). The findings reported here are similar to those from studies in nearby areas, i.e. Mexico and Belize (Castelblanco-Martinez et al., Reference Castelblanco-Martinez, Morales-Vela, Hernandez-Arana and Padilla-Saldivar2009; Flores-Cascante et al., Reference Flores-Cascante, Morales-Vela, Castelblanco-Martinez, Padilla-Saldivar and Auil2013), and are comparable to studies conducted in similar habitats around Puerto Rico (Mignucci-Giannoni & Beck, Reference Mignucci-Giannoni and Beck1998). Manatees feed predominantly on seagrasses, as determined in previous studies, and the seagrasses consumed by manatees in this study are in similar order of importance as reported by Mignucci-Giannoni & Beck (Reference Mignucci-Giannoni and Beck1998), Castelblanco-Martinez et al. (Reference Castelblanco-Martinez, Morales-Vela, Hernandez-Arana and Padilla-Saldivar2009) and Flores-Cascante et al. (Reference Flores-Cascante, Morales-Vela, Castelblanco-Martinez, Padilla-Saldivar and Auil2013). The findings are also congruent with results from stable isotope analysis of manatee tissues from Belize (Alves-Stanley et al., Reference Alves-Stanley, Worthy and Bonde2010). Other vascular plant material (mangrove specifically) made up a considerable portion of the identified diet items. Similar findings have been reported for manatees in Puerto Rico, Mexico and Belize (Mignucci-Giannoni & Beck, Reference Mignucci-Giannoni and Beck1998; Castelblanco-Martinez et al., Reference Castelblanco-Martinez, Morales-Vela, Hernandez-Arana and Padilla-Saldivar2009; Flores-Cascante et al., Reference Flores-Cascante, Morales-Vela, Castelblanco-Martinez, Padilla-Saldivar and Auil2013). Fragments of Ulva sp., Chara sp., and Lyngbya sp. were identified, but unidentified algal remains were a frequent occurrence. Of the three discernible algal species, two have been reported from Antillean manatees in other locations: Ulva sp. in Puerto Rico (Mignucci-Giannoni & Beck, Reference Mignucci-Giannoni and Beck1998) and Chara sp. in Mexico (Castelblanco-Martinez et al., Reference Castelblanco-Martinez, Morales-Vela, Hernandez-Arana and Padilla-Saldivar2009). All three algal species have been observed in content samples from Florida (Hurst & Beck, Reference Hurst and Beck1988). Manatee diet samples in Belize also contained small amounts of invertebrates, primarily identified as an unknown poriferan.
Invertebrates often occur in close association with seagrasses and incidental ingestion is likely (Hartman, Reference Hartman1979; Best, Reference Best1981; Ledder, Reference Ledder1986; Hurst & Beck, Reference Hurst and Beck1988; Courbis & Worthy, Reference Courbis and Worthy2003), although manatees and dugongs are known to be omnivorous when resources are limited (Lipkin, Reference Lipkin1976; Powell, Reference Powell1978; Preen, Reference Preen1995a). Sand grains, detrital material and microalgae were observed in the samples we examined and were probably ingested incidentally. Mouth and stomach samples were the least digested and contained the most identifiable fragments. Manatees have a very long digestive tract and a 4–7 day passage time (Larkin et al., Reference Larkin, Fowler and Reep2007). Faecal samples, which are more fully digested, were the greatest challenge for identification. Faecal samples therefore may not precisely reflect individual feeding habits, since some components of the diet may no longer be identifiable. As expected, faecal samples contained a higher amount of unknown or unidentifiable points during examination and contained a higher percentage of rhizomes which is not as easily degraded through digestion. Because some portions of the samples contained unidentified fragments, the complete composition of each sample could not be attained. For example, not all algae were identifiable, but filamentous algae were more often observed in our Belize samples.
Diet differences between locations
Seagrass was found in more samples and in higher percentages in samples collected in Southern Lagoon (P = 0.03); algae and mangrove made up a larger proportion of those samples from the Drowned Cayes (P = 0.03). Likewise, Alves-Stanley et al. (Reference Alves-Stanley, Worthy and Bonde2010) observed a difference in δ13C and δ15N values between manatees sampled in the Drowned Cayes and Southern Lagoon. Observed variation in ingested food items between manatees in Southern Lagoon and Drowned Cayes may be attributed to environmental differences between the two sites.
As manatees are able to move between fresh, salt and brackish waters, they have been documented to make use of dominant plant types in each type of environment. Differences in diet between the two locations in Belize illustrated only slight dietary differences. By comparison, manatee diets in Florida are highly variable by region (Alves-Stanley et al., Reference Alves-Stanley, Worthy and Bonde2010). Florida manatee diets often contain the same seagrass types as manatees in the Caribbean (Best, Reference Best1981; Ledder, Reference Ledder1986; Hurst & Beck, Reference Hurst and Beck1988; Mignucci-Giannoni & Beck, Reference Mignucci-Giannoni and Beck1998; Castelblanco-Martinez et al., Reference Castelblanco-Martinez, Morales-Vela, Hernandez-Arana and Padilla-Saldivar2009; Flores-Cascante et al., Reference Flores-Cascante, Morales-Vela, Castelblanco-Martinez, Padilla-Saldivar and Auil2013), but also contain several additional genera of algae and freshwater plants not identified in these Belize samples (e.g. Chara, Gracilaria, Hydrilla, Myriophyllum, Najas, Vallisneria). Antillean manatee diet samples in Brazil were comprised of 21 different types of algae, seagrasses and invertebrates, but predominantly contained rhodophytes (Borges et al., Reference Borges, Araujo, Anzolin and Miranda2008), while Amazonian manatees consumed at least 24 different plant species within the central Amazon (Borges et al., Reference Borges, Araujo, Anzolin and Miranda2008).
The presence of mangrove fragments detected in the samples obtained from manatees in the Drowned Cayes (59%) was higher than those for individuals sampled from Southern Lagoon (51%). In the Drowned Cayes, manatees were usually associated with, and captured near, mangrove forest islands located offshore of the mainland. Mangrove islands provide shelter for manatees in these open-water marine environments, and additionally, mangrove leaves store fresh water (Odum & McIvor, Reference Odum, McIvor, Myers and Ewel1990). The higher detection of mangrove ingesta in the manatees from the Drowned Cayes may indicate, by association, the use of mangrove forage to supplement their need for fresh water. The fresh water available in this vascular plant may enable manatees to spend longer periods of time in marine environments.
Diet differences between sexes and sizes
There were no significant differences observed in diet preferences between male and female manatees in Belize. This finding is consistent with a previous study carried out in Mexico (Castelblanco-Martinez et al., Reference Castelblanco-Martinez, Morales-Vela, Hernandez-Arana and Padilla-Saldivar2009). No other known published account has demonstrated a significant difference in diet between the sexes. Of the 76 females in our study, four were sampled when lactating. Pregnant or lactating females may have additional nutritional needs, but no obvious difference in diet composition of these females was discernible.
No difference in manatee diet between size classifications was detected for seagrass or [other] vascular plant consumption, but adult manatees (>245 cm) consumed a statistically greater proportion of algae: adult mean 3.8 ± 0.65, juveniles 2.3 ± 0.89 (P = 0.04). No previous studies have analysed the relationship between manatees' size (length) and diet. Influencing factors may be a result of larger manatees consuming a greater amount of food and/or the duration of time spent feeding.
Diet differences between seasons
Manatees in Belize have been observed to utilize different habitat types in the wet and dry seasons (Morales-Vela et al., Reference Morales-Vela, Olivera-Gomez, Reynolds and Rathbun2000; Self-Sullivan et al., Reference Self-Sullivan, Smith, Packard and LaCommare2003; Auil, Reference Auil2004; Auil et al., Reference Auil, Powell, Bonde, Andrewin and Galves2007), however, no statistical differences in the diet samples were observed in this study. Similar to Belize, no seasonal differences in diet were observed nearby in Chetumal Bay, Mexico (Castelblanco-Martinez et al., Reference Castelblanco-Martinez, Morales-Vela, Hernandez-Arana and Padilla-Saldivar2009), and no seasonal differences in diet have been observed in the dugong as well (Preen, Reference Preen1995b). In the Amazon, manatees have been observed to be more selective during the rainy season, but fed on a more diverse number of plants during the dry season (Colares & Colares, Reference Colares and Colares2002; Guterres-Pazin et al., Reference Guterres-Pazin, Marmontel, Rosas, Pazin and Venticinque2014). Although seasonal habitat use may change within Belize, the uniform similarity of food plants available for consumption year-round may account for the lack of seasonal diet differences reported in this study.
Alves-Stanley et al. (Reference Alves-Stanley, Worthy and Bonde2010) used stable-isotopes to infer seasonal changes in manatee diet and observed a difference in δ13C and δ15N values between manatees sampled in the Drowned Cayes and Southern Lagoon. Rather than attribute this simply to environmental differences between the two sites, this could be a result of changes in manatee resource selection (food or habitat use), and/or seasonal variability in the stable isotope ratios of vegetation. Manatees in Belize have been known to make seasonal changes in habitat use (Morales-Vela et al., Reference Morales-Vela, Olivera-Gomez, Reynolds and Rathbun2000; Auil et al., Reference Auil, Powell, Bonde, Andrewin and Galves2007) and have been documented moving between the two sampling sites within the same day (RKB, JAP, personal observation). As most of the specimens examined in this study were faecal samples, a precise snapshot of manatee diet differences cannot be assumed due to a manatees' long gut transit time.
Conclusions and future recommendations
Belize is a central location for the Antillean manatee population (O'Shea & Salisbury, Reference O'Shea and Salisbury1991) and provides important habitat for the long-term survival of the subspecies (Quintana-Rizzo & Reynolds, Reference Quintana-Rizzo and Reynolds2007; Auil Gomez, Reference Auil Gomez, Palomares and Pauly2011). Manatee populations can be used to assess the health of marine ecosystems as they are a sentinel species for habitat quality (Bonde et al., Reference Bonde, Aguirre and Powell2004). In Belize, manatees face an increasing threat from anthropogenic and natural causes of mortality including boat strikes and habitat alteration from construction and contaminated effluents (Auil Gomez, Reference Auil Gomez, Palomares and Pauly2011). An understanding of the types, species, areas and feeding preferences of manatees is helpful for developing conservation and habitat protection plans for manatees.
This study found that the seagrasses Halodule wrightii, Thalassia testudinum and Ruppia maritima are the predominant food items consumed by manatees in Belize. Red mangrove (Rhizophora mangle) also made up a significant portion of the observed manatee diet. Algae and invertebrates were detected in small quantities. The high frequency of seagrass rhizome and mangrove leaves observed in the faecal samples may be attributed to the indigestibility and toughness of these fragments, and, therefore, the overall proportions may be overrepresented. Further, the amount of rhizome consumed is dependent on the species and substrate. Our findings emphasize the importance for protection and preservation of seagrass and mangrove habitats in Belize to promote a positive outcome for this important regional population of manatees.
ACKNOWLEDGEMENTS
This research was conducted as part of a Master of Science thesis project of ACA while at Nova Southeastern University. ACA would like to thank his committee members Dr James D. Thomas and Dr Curtis M. Burney of Nova Southeastern University, and CAB and RKB of USGS for their encouragement and support. We appreciate the assistance of the Belize Marine Mammal Stranding Network, in particular Jamal Galves of Sea to Shore Alliance-Belize, and thank the capture team from Gales Point ‘Manatee’ Village for enabling the collection of the samples used in this study. Additional thanks to Dr Donald W. Ott at the University of Akron for confirmation of diet contents through electron light microscopy. We appreciate the constructive and insightful comments of Dr Tom Frazer, University of Florida, and two anonymous reviewers on earlier versions of this manuscript. This work was conducted under authority granted by the Belize Forestry Department, as well as the U.S. Fish and Wildlife Service research permit MA791721-4 issued to the U.S. Geological Survey Sirenia Project. All sample collection was conducted in accordance with IACUC standards and samples were imported into the USA under valid CITES permits. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Appendix A. Per cent frequency: location.
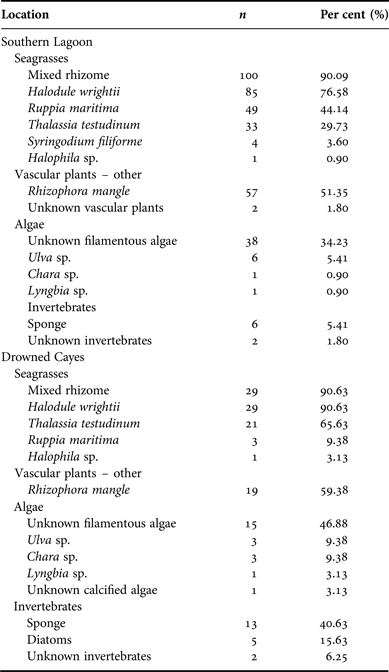
Appendix B. Belize manatee diet sample composite: location.
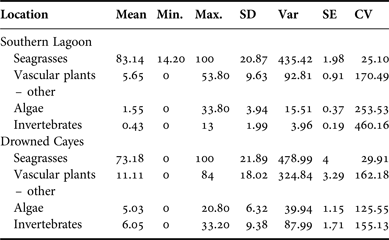
Appendix C. Per cent frequency: sex.

Appendix D. Belize manatee diet sample composite: sex.
Appendix E. Per cent frequency: size classification.
Appendix F. Belize manatee diet sample composite: size classification.
Appendix G. Per cent frequency: season.
Appendix H. Belize manatee diet sample composite: season.