Purpose
Electronic brachytherapy (eBT) has been evolving since the turn of the centuryReference Dickler and Dowlatshahi 1 and has become a treatment option for different types of tumour locations in various scenarios.Reference Vaidya, Baum, Tobias, Morgan and De Souza 2 – Reference Richardson, García-Ramírez, Lu, Myerson and Parikh 6 The Axxent eBT unit (Xoft, Inc., an iCAD subsidiary, San José, CA, USA) provides treatment to patients with a 50 kVp miniature X-ray source that directly irradiates the tumour site in skin cancer and with different applicators in the case of breast or gynaecological cancers. This unit allows treatment of skin tumour lesions (nonmelanoma), performance of intraoperative breast radiotherapy and postoperative endometrial and cervical cancer treatments. Specifically, it can also be used to treat local and locally advanced endometrial cancer in protocols that require high absorbed dose rate (HDR) after a hysterectomy and/or external radiotherapy.Reference Dooley, Thropay and Schreiberg 7 At our centre, the eBT system was acquired in May 2015 to be used in skin locations and in intraoperative radiotherapy procedures for breast cancer after lumpectomy. From September 2015, postoperative endometrial cancer treatments began and were carried out with Axxent eBT unit. Traditionally, this type of treatment has been carried out in many centres throughout the world with HDR equipment with Iridium-192 (Ir-192, 73.8-day half-life and average energy of 0.355 MeV) sources that irradiate the vaginal vault by a cylindrical applicator of an appropriate size for each case (generally, the largest size possible, given the patient’s anatomy).Reference Gerbaulet, Pötter, Mazeron, Meertens and Van Limbergen 8 Other sources have been used for this purpose, such as Cobalt-60 (Co-60, 5.27-year half-life and average energy of 1.25 MeV),Reference Gerbaulet, Pötter, Mazeron, Meertens and Van Limbergen 8 with the advantage that there are fewer source changes; therefore, it is more economical, although each brachytherapy session is longer.
Previous published studies for patients treated with Ir-192 compared endometrium,Reference Mobit, Nguyen, Packianathan, He and Yang 9 , Reference Dickler, Kirk and Coon 10 cervixReference Mobit, Packianathan, He and Yang 11 and breast,Reference Ahmad, Johnson and Hiatt 12 and always showed a lower dose in the organs at risk (OAR) for patients planned with eBT. In all these studies patients were treated with Ir-192, but in our work patients are treated with eBT and we report the monitoring of early toxicity as one of the main objectives of this research.
The aim of this study was to undertake a dosimetric comparison of traditional HDR brachytherapy and eBT to ensure the safety and results in terms of toxicity in OAR. The objective of treatment with eBT is to provide an alternative to the aforementioned radioactive sources with a portable unit, with the resulting advantage of mobility, absence of room shielding (for this energy, an 0.5 mm Pb-equivalent shield in walls and doors is sufficient), avoiding the need to transport and change radioisotope sources and their ease of use.Reference Eaton, González, Duck and Keshtgar 13
Materials and Methods
A total of 94 patients were treated with the Axxent eBT unit from September 2015 to October 2017 using cylindrical applicators of sizes ranging from 2.5 to 3.5 cm in diameter, with active lengths of 2.5 to 3.5 cm (Table 1). Each eBT source has a working life of 500 min of clinical use, discounting the quality assurance time; each endometrium session lasted between 3 and 4 min. If a source is not stable or when 500 min of treatment have been reached, it is removed and replaced (Table 2). Ethical approval was gained from Research Ethics Committee of the Autonomous Community of Aragon for the study. The mean age of patients was 65.9 years (33–84 years), with different tumour staging (Table 3). All patients presented with endometrial cancer during this timeframe. Of the 94 patients, 37 received exclusive adjuvant eBT [stage IA cases according to Fédération Internationale de Gynécologie et d’Obstétrique (FIGO) and seven patients with IB1 FIGO stage] (25 Gy in five sessions); the remaining patients received adjuvant external beam radiotherapy (EBRT) with a regimen of 23 sessions of 2 Gy each to the entire pelvis, followed by eBT (15 Gy in three sessions). Sessions were administered twice weekly (Monday and Friday) after EBRT treatment ends. In patients treated with EBRT and eBT, the time from EBRT and the end of eBT is 58.1 days (35–78 days), and the time between the end of radiotherapy and the end of eBT is 14.7 days (6–35 days) (Table 3). Before radiotherapy, 33 patients (35%) received chemotherapy treatment and 61 did not (65%). Calculations made for the patients are compared with the calculations made a posteriori for Ir-192 and Co-60 sources for each of the 94 patients, giving a total of 282 different plans. For eBT, each patient underwent a computed tomography study with sections every 3 mm before the first session. The different OARs of interest were contoured; in this case, the urinary bladder, rectum and sigmoid colon, following the recommendations of the American Brachytherapy Society.Reference Nack, Eriksson, Parikh, Gupta, Varia and Glasgow 14 Then the planning target volume (PTV) was contoured and defined by the cylinder along its active length plus a 5 mm margin with the cylinder volume removed from the PTV. The objective is to provide the prescribed dose at 5 mm from the applicator throughout its active length.Reference Nack, Eriksson, Parikh, Gupta, Varia and Glasgow 14 After the medical physicist prepared the plan, the radiation oncologist approved it, and if appropriate, the patient was treated. Subsequently, two more plans were produced for treatment with Ir-192 and Co-60 sources, respectively.Reference Nath, Anderson, Luxton, Weaver, Williamson and Meigooni 15 – Reference Hiatt, Rivard and Hughes 18 The units that we defined in the treatment planning system (TPS) for this purpose are Gammamed Plus (Varian Medical System, Inc.) for the Ir-192 and Eckert & Ziegler Bebig for calculations with Co-60. TG-43 is the algorithm available in our TPS, with which we perform all calculations. In data referring to the PTV, the values of the dose at 90% of the PTV (D 90) and of the volume that receives 100% of the dose (V 100) have been determined, but will not be shown in this research, as they are the same in the three treatment plans produced, and the same standardisation is implemented in all planning. The V 150 and V 200 were also determined. With regard to the OAR, we determined the same parameters for all OAR of interest in the study: urinary bladder, rectum and sigmoid colon. The parameters compared are D 2cc (maximum dose at 2 cc of volume), V 50% (volume up to 50% of the prescribed dose) and V 35% (volume up to 35% of the prescribed dose) and assessed toxicity according to the Radiation Therapy Oncology Group (RTOG) parameters.Reference Cox, Stetz and Pajak 19
Table 1 Cylinder size and active lengths
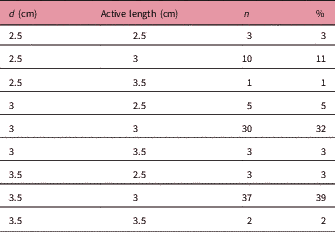
Abbreviations: d: cylinder diameter; n: number of patients.
Table 2 Characteristics of electronic brachytherapy sources

Table 3 Patient and treatment characteristics
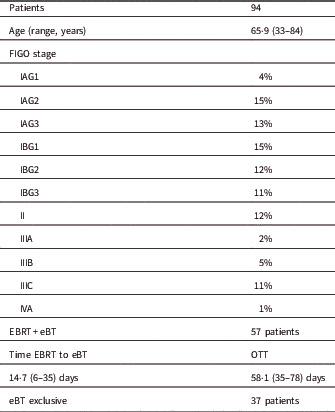
Notes: Proportions are given with number of total and median range. FIGO stage is given in percentage of the total number of patients.
Abbreviations: eBT, electronic brachytherapy treatment; Time EBRT to BQ, time between the end EBRT and the start of eBT; OTT, overall treatment time; Total time: time from the start of the EBRT to the end of the eBT.
Results
The mean follow-up of patients was 14 months (4–25). The dosimetry results obtained for patients planned with eBT show much lower doses in OAR than those planned with Ir-192. The mean dose parameters of all treatments compared showed that the bladder D 2cc for eBT was 63.8% versus 70.1% for HDRBT Ir-192 with the difference in the V 50% (7.2% versus 12.7%) and V 35% (15.2% versus 28.2%) being much more remarkable.
Also, in the rectum we observed 61.2% versus 68.4% in D 2cc, 7.9% versus 14.3% in V 50% and 16.7% versus 32% in V 35%. The results for HDRBT Co-60 were similar to those obtained for Ir-192 (Table 4).
Table 4 Mean values of dosimetric parameters
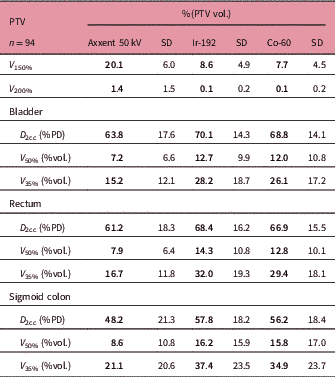
Abbreviations: PTV, planning target volume; V 150 and V 200, percentage of the PTV receiving 150% and 200% of the prescribed dose; D 2cc, maximum dose of 2 cc; %PD, percentage of the prescribed dose; V 50% and V 35%, percentage of organ receiving 50% or 35% of the prescribed dose.
The D 90 of each plan was not included in the results, as it was the value used to standardize each plan. The V 150 and the V 200 were higher for cases calculated (and treated) with Axxent than for those calculated with Ir-192 or Co-60, although this difference decreases as the cylinder size increases (Table 5). Acute mucositis cases in patients have not been observed: of the 94 patients treated in our centre, only one presented grade 2 (RTOG) acute toxicity (Table 6).Reference Cox, Stetz and Pajak 19 One month after treatment, the patients’ vaginal toxicity was grade 0 (RTOG) for 94.7% of the patients and grade 1 for the remaining 5.3%, with grade 2 cases completely disappearing. Rectal toxicity grade 0 was 98.9% and grade 1 was 1.1%, and urinary toxicity grade 0 was 97.9% and grade 1 was 2.1% (Table 7). We observed that patients for whom 12 months have already passed since treatment ended, that is 31 patients, and median follow-up of 19 months (12–25 months) did not present any recurrence. The prescribed dose in each eBT treatment was implemented without considering that we administered treatment with a much lower average beam energy than in the case of Ir-192 (26 keV compared with 355 keV) and therefore, without considering the differences in the different relative radiobiological effectiveness (RBE) expected for low-energy irradiation.Reference Reniers, Liu, Rusch and Verhaegen 20 – Reference White, Reniers, de Jong, Rusch and Verhaegen 22
Table 5 Mean values per cylinder size
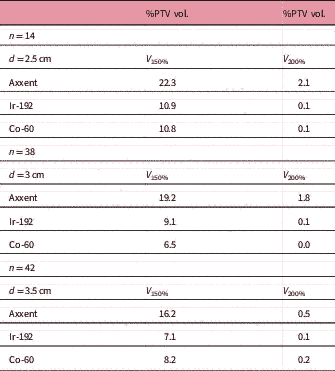
Abbreviations: n, number of patients; d, cylinder diameter; V 150 and V 200: percentage of the PTV receiving 150% and 200% of the prescribed dose.
Table 6 Acute toxicity according to RTOG-EORTC

Note: Grades 0,1,2.Reference Cox, Stetz and Pajak 19
Abbreviation: n, number of patients.
Table 7 Toxicity one month after eBT treatment for all 94 patients according to RTOG-EORTC

Note: Grades 0,1,2.Reference Cox, Stetz and Pajak 19
Abbreviation: n: number of patients.
Discussion
Results were obtained in patients treated after the first 25 months, with median follow-up of 14 months (4–25 months), on the clinical use of the Axxent eBT unit for postoperative treatment of endometrial cancer, indicating why this option is a good alternative.
It is possible to obtain the same vaginal vault coverage as in the planning when using Ir-192 or Co-60, and reduction of doses to OAR is achieved although the V 150 and the V 200 of the PTV is increased, which could have produced an increase in acute mucositis cases in our patients but the results of toxicity, together with the dose reduction to OAR, lead us to conclude that eBT is a good alternative to treatments with Ir-192 or Co-60. In another study of only 10 patients treated with Ir-192 and Co-60 and comparing the treatment plans with eBT (10) results reported a difference in rectum for D 2cc between eBT and Ir-192 (86.7% versus 88.3% of prescribed dose) but similar results for V 35% (36.9% versus 58.9%) and V 50% (20.4 versus 32.7); for the bladder the D 2cc was 43.3% for eBT and 55% of the dosage prescribed for Ir-192 with V 35% (37.9 versus 72.3) and V 50% (15.6 versus 33.9). In a multicentre study of patients treated with eBT,Reference Dickler, Puthawala, Thropay, Bhatnagar and Schreiber 23 the results are similar to this study: dose to the bladder at V 50% of 11.5±9.7% and rectum 17.4±10.9% in eBT plans.
Considering these results, the authors believe that eBT is a good option for a radiation oncology department without a brachytherapy unit (since additional research is needed with longer follow-up) for the treatment of endometrial cancer, given the mobility, versatility and ease of installation of the equipment combined with appropriate dosimetry results and very low toxicity in patients, according to the initial results shown. In the absence of a longer clinical follow-up, the results are highly promising. With regard to the calculation method used, it is recommendedReference Beaulieu, Tedgren and Carrier 24 to consider the composition of the tissues and perform calculations based on Monte-Carlo models. Our calculations are performed with TG-43 without correction for heterogeneity because it is the algorithm available in our centre and in many other hospitals for which these results will be useful. Although Monte-Carlo methods have been compared with the clinical results of other radionuclides, Reniers et al. came to the conclusion that eBT source has similar RBE as the 125I isotope,Reference Reniers, Liu, Rusch and Verhaegen 20 for Axxent eBT, the published RBE values are still pending clinical verification.Reference Shi, Guo, Cheng, Eng and Papanikolaou 25 Studies have been done to determine the RBE of 50 keV eBT sources used in this type of treatment, where RBE factors are calculated for different tissues. RBE calculated by Brenner et al. varies between 1.29 and 1.85 with respect to the Ir-192 to 5 mm depth.Reference Brenner, Leu, Beatty and Shefer 21 Publications, after carefully reviewing the prescription of treatments according to these factors,Reference Brenner, Leu, Beatty and Shefer 21 , Reference Shi, Guo, Cheng, Eng and Papanikolaou 25 , Reference Rava, Dvorak and Markelewicz 26 always recommend that this be done with caution and based on clinical studies; following these recommendations, we should have reduced the prescribed dose to prevent toxicities, and we may have reduced local control. A clinical study showed the reduction of the prescribed dose in the treatment of nodular and superficial basal cell carcinoma, using eBT, based on RBE decreased tumour control from 95 to 90%, showing more tumour control for standard prescription.Reference Ballester-Sánchez, Pons-Llanas and Candela-Juan 27 In reference to the postoperative endometrial cancer treatment, Rava et al. showed a dose decrease in the urinary bladder and rectum, taking into account the equivalent biological dose for an α/β=3 (BED3) with eBT, which may be related to a decrease of late toxicity in these organs; all of this was calculated with an RBE factor of 1.5. However, in the same study, there was a dose increase on the surface of the mucosa if we calculate for this same RBE the equivalent doses BED10 and BED3, which would give an overdose to the vaginal vault and higher toxicity of the mucose. Rava et al. suggest considering de-escalation of the dose to account for the differences in RBE,Reference Rava, Dvorak and Markelewicz 26 again recommending more clinical studies to consider. Therefore, more studies must be conducted, and more improvements must be made in dosimetry calculation with eBT to compensate for the difference in RBE, although there are important uncertainties in the estimation of this parameter, which imply that the results may vary from one publication to another; it is a subject for discussion.Reference Eaton 28 In our case, not using corrections of the RBE has not caused an increase of mucositis or any other toxicity or inadequate local control (only 30 patients had a 12 months follow-up). This study represents the largest published series of patients with endometrial cancer treated with eBT to date, showing excellent results in terms of early effects in all patients with a much lower dose in OAR than in patients treated with sources of Ir-192 or Co-60. Higher values of V 150 and V 200 in the case of eBT do not result in higher cases of toxicity to the vaginal mucosa. The dose differences in the OARs are very marked, especially in the V 35% and V 50% are analysed.
Conclusion
The eBT treatments are a great advantage in centres without HDR equipment, although more clinical and local control results with longer follow-up are needed. The eBT equipment is a useful addition to centres with HDR equipment, as a complimentary facility due to its mobility and versatility in being able to perform treatments for endometrial cancer, intraoperative breast radiotherapy, skin cancer and even treatments of cervical cancer in selected cases.
Acknowledgement
None.











