Article contents
Thio-oxynitride phosphate glass electrolytes prepared by mechanical milling
Published online by Cambridge University Press: 21 May 2015
Abstract
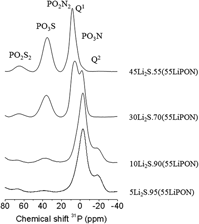
Lithium thio-phosphorus oxynitride glasses, LiPOSN, have been prepared by mechanical milling process from the mixture of Li2S and LiPON glass. The anionic substitution of oxygen by sulphur and nitrogen in the phosphate glass structure has been confirmed by 1D 31P solid state nuclear magnetic resonance and x-ray photoelectron spectroscopy. The study of thermal and electrical properties reveals a decrease in the glass transition temperature, likely due to the depolymerization of glass network by the decrease of bridging oxygens and sulphurs, along with a sharp increase in the ionic conductivity when lithium sulphide is incorporated into the oxynitride glasses. The improvement of chemical durability by the introduction of nitrogen, together with the increase in ionic conductivity up to values closed to the value of commercial LiPON thin film electrolyte, suggests that these LiPOSN glasses could be good candidates as solid electrolytes for lithium microbatteries.
- Type
- Articles
- Information
- Journal of Materials Research , Volume 30 , Issue 19: Focus Issue: Nitrides and Oxynitride Materials , 14 October 2015 , pp. 2940 - 2948
- Copyright
- Copyright © Materials Research Society 2015
References
REFERENCES
- 9
- Cited by




