Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Cockreham, Cody B.
Zhang, Xianghui
Lau, Miu Lun
Long, Min
Guo, Xiaofeng
Xu, Hongwu
and
Wu, Di
2020.
Thermal Evolutions and Resulting Microstructural Changes in Kerogen-Rich Marcellus Shale.
ACS Earth and Space Chemistry,
Vol. 4,
Issue. 12,
p.
2461.
Kříbek, Bohdan
Bičáková, Olga
Sýkorová, Ivana
Havelcová, Martina
Veselovský, František
Knésl, Ilja
and
Mészárosová, Noemi
2021.
Experimental pyrolysis of metalliferous coal: A contribution to the understanding of pyrometamorphism of organic matter and sulfides during coal waste heaps fires.
International Journal of Coal Geology,
Vol. 245,
Issue. ,
p.
103817.
Tang, Xinhua
Cui, Yang
and
Liu, Lei
2021.
Pyrolyzing pyrite and microalgae for enhanced anode performance in microbial fuel cells.
International Journal of Hydrogen Energy,
Vol. 46,
Issue. 75,
p.
37460.
Moreau, Juulia‐Gabrielle
Jõeleht, Argo
Aruväli, Jaan
Heikkilä, Mikko J.
Stojic, Aleksandra N.
Thomberg, Thomas
Plado, Jüri
and
Hietala, Satu
2022.
Bulk synthesis of stoichiometric/meteoritic troilite (FeS) by high‐temperature pyrite decomposition and pyrrhotite melting.
Meteoritics & Planetary Science,
Vol. 57,
Issue. 3,
p.
588.
Wang, Xuelian
Zhang, Xuekai
Tong, Peng
Yang, Cheng
Si, Jianguo
Xiong, Tingjiao
Dong, Buke
Xie, Lulu
Pan, Chengbing
Wang, Meng
Lin, JianChao
Chen, Huaican
Yin, Wen
Song, Wenhai
and
Sun, Yuping
2022.
Latent Heat Thermal Storage of Solid-State Phase Transition in Thermally Stabilized Hexagonal FeS.
SSRN Electronic Journal ,
Zhang, Xiaoliang
Zhu, Yangge
Sun, Chunbao
and
Kou, Jue
2022.
The mechanism of microwave-induced phase transformation and sulfur conversion in gold-bearing pyrite under inert atmospheres.
Minerals Engineering,
Vol. 186,
Issue. ,
p.
107742.
Lee, Sugyeong
Sadri, Farzaneh
and
Ghahreman, Ahmad
2022.
Enhanced Gold Recovery from Alkaline Pressure Oxidized Refractory Gold Ore After its Mechanical Activation Followed by Thiosulfate Leaching.
Journal of Sustainable Metallurgy,
Vol. 8,
Issue. 1,
p.
186.
Wang, Xuelian
Zhang, Xuekai
Tong, Peng
Yang, Cheng
Si, Jianguo
Xiong, Tingjiao
Dong, Buke
Xie, Lulu
Pan, Chengbing
Wang, Meng
Lin, JianChao
Chen, Huaican
Yin, Wen
Song, Wenhai
and
Sun, Yuping
2022.
Latent Heat Thermal Storage of Solid-State Phase Transition in Thermally Stabilized Hexagonal FeS.
SSRN Electronic Journal ,
Larachi, Faïçal
Lukumu, David Bampolé
and
Baş, Ahmet Deniz
2023.
Susceptibility to cyanidation of pyrrhotite-associated gold in pyrite calcines from (non)oxidizing roasting environments.
Minerals Engineering,
Vol. 202,
Issue. ,
p.
108245.
Liu, Jinting
Zhang, Weifang
Chen, Xiaoduo
Huang, Zijian
Fu, Xiaoheng
and
Wang, Chunli
2023.
Effect of pyrite packed thickness on its oxidation pathway in high temperature.
Energy Sources, Part A: Recovery, Utilization, and Environmental Effects,
Vol. 45,
Issue. 1,
p.
1874.
Chaney, Donald Z.
Hirtz, John
Williams, Evan
Minnette, Jacob
Cureton, William F.
O’Quinn, Eric C.
Zhao, Xiaodong
Guo, Xiaofeng
Matsuoka, Takahiro
Koehler, Michael
Sprouster, David
and
Lang, Maik
2023.
Grain size dependence of thermally induced oxidation in zirconium carbide.
Journal of Materials Science,
Vol. 58,
Issue. 6,
p.
2439.
Elsadek, Mohamed
Ahmed, Hesham
Suup, Malin
Sand, Anders
Heikkinen, Eetu
Khoshkhoo, Mohammad
and
Sundqvist-Öqvist, Lena
2023.
Recycling of pyrite and gypsum mining residues through thermochemical conversion into valuable products.
Resources, Conservation and Recycling,
Vol. 199,
Issue. ,
p.
107219.
Wang, Xuelian
Zhang, Xuekai
Tong, Peng
Yang, Cheng
Si, Jianguo
Xiong, Tingjiao
Dong, Buke
Xie, Lulu
Pan, Chengbing
Wang, Meng
Lin, Jianchao
Chen, Huaican
Yin, Wen
Song, Wenhai
and
Sun, Yuping
2023.
Latent heat thermal storage of solid-state phase transition in thermally stabilized hexagonal FeS.
Scripta Materialia,
Vol. 225,
Issue. ,
p.
115166.
Klyushnikov, A. M.
Gulyaeva, R. I.
Pikalov, S. M.
and
Maltsev, G. I.
2023.
Kinetics and mechanism of oxidizing roasting of sulfide copper-cobalt ore.
iPolytech Journal,
Vol. 27,
Issue. 1,
p.
188.
Azimov, Farkhod
Lee, Jinseok
Park, Subin
and
Jung, Hyun Min
2023.
Fabrication of Assembled FeS2 Nanosheet and Application for High-Performance Supercapacitor Electrodes.
ACS Applied Materials & Interfaces,
Vol. 15,
Issue. 22,
p.
26967.
Klyushnikov, Alexander M.
Pikalov, Sergey M.
and
Gulyaeva, Roza I.
2023.
Kinetics of solid-state oxidation of iron, copper and zinc sulfide mixture.
Chimica Techno Acta,
Vol. 10,
Issue. 2,
Qian, Weilun
Zhang, Huibin
Tan, Jiankang
Feng, Wenyu
Cao, Huazhen
and
Zheng, Guoqu
2024.
Reactive synthesis of ferrous sulfide using elemental iron/pyrite ore: Kinetics study and application.
Minerals Engineering,
Vol. 208,
Issue. ,
p.
108579.
Parayangattil Jyothibasu, Jincy
Tien, You-Ching
Chen, Zi-Ting
Yang, Hongta
Chiang, Tzu Hsuan
EL-Mahdy, Ahmed F. M.
and
Lee, Rong-Ho
2024.
Iron Sulfide Microspheres Supported on Cellulose-Carbon Nanotube Conductive Flexible Film as an Electrode Material for Aqueous-Based Symmetric Supercapacitors with High Voltage.
ACS Omega,
Vol. 9,
Issue. 24,
p.
26582.
Muñoz-Cortés, E.
Sánchez-Prieto, J.
Zabala, B.
Sanchez, C.
Flores, E.
Flores, A.
Roman, E.
Ares, J. R.
and
Nevshupa, R.
2024.
Operando exploration of tribochemical decomposition in synthetic FeS2 thin film and mineral iron pyrite.
RSC Mechanochemistry,
Vol. 1,
Issue. 2,
p.
196.
Rivera Li Kao, Oscar
and
Garbers-Craig, Andrie
2024.
Decomposition of Sulfide Phases and Subsequent Matte Collection in the Black Top of a Platinum Group Metal Smelter.
Mineral Processing and Extractive Metallurgy Review,
p.
1.
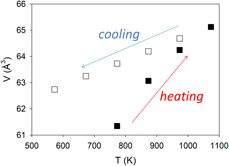
 $\bar 3$) to be αV = 3.7456 × 10−5 K−1, which largely results from the expansion of the Fe–S bond. With further increase in temperature to 1073 K, all the pyrite transformed to pyrrhotite (Fe1−xS) at 873 K. Unit-cell parameters of Fe1−xS (space group P63/mmc) increase on heating and decrease on cooling. However, the rates in cell expansion are larger than those in contraction. This hysteresis behavior can be attributed to continuous desulfurization of pyrrhotite (i.e., x in Fe1−xS decreases) with increasing temperature until the stoichiometric troilite (FeS) was formed at 1073 K. On cooling, troilite underwent a magnetic transition to an orthorhombic structure (space group Pnma) between 473 and 573 K. In addition, using differential thermal analysis (DTA) and thermogravimetric analysis (TGA) implemented with a differential scanning calorimeter, we performed kinetic measurements of pyrite decomposition. Detailed peak profile and Arrhenius (k = A exp(−Ea/RT)) analyses yielded an activation energy Ea of 302.3 ± 28.6 kJ/mol (based on DTA data) or 302.5 ± 26.4 kJ/mol (based on TGA data) and a ln(A) of 35.3 ± 0.1.
$\bar 3$) to be αV = 3.7456 × 10−5 K−1, which largely results from the expansion of the Fe–S bond. With further increase in temperature to 1073 K, all the pyrite transformed to pyrrhotite (Fe1−xS) at 873 K. Unit-cell parameters of Fe1−xS (space group P63/mmc) increase on heating and decrease on cooling. However, the rates in cell expansion are larger than those in contraction. This hysteresis behavior can be attributed to continuous desulfurization of pyrrhotite (i.e., x in Fe1−xS decreases) with increasing temperature until the stoichiometric troilite (FeS) was formed at 1073 K. On cooling, troilite underwent a magnetic transition to an orthorhombic structure (space group Pnma) between 473 and 573 K. In addition, using differential thermal analysis (DTA) and thermogravimetric analysis (TGA) implemented with a differential scanning calorimeter, we performed kinetic measurements of pyrite decomposition. Detailed peak profile and Arrhenius (k = A exp(−Ea/RT)) analyses yielded an activation energy Ea of 302.3 ± 28.6 kJ/mol (based on DTA data) or 302.5 ± 26.4 kJ/mol (based on TGA data) and a ln(A) of 35.3 ± 0.1.



