Article contents
Synthesis and study of carbon/TiO2 and carbon/TiO2 core–shell micro-/nanospheres with increased density
Published online by Cambridge University Press: 29 October 2012
Abstract
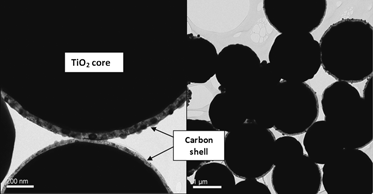
Nanosized graphitic carbon provides high selectivity and stability of catalysts. However, the carbon is very light when used as a support, even when loaded with metals. To counteract this problem, carbon/TiO2 core–shell structures were synthesized via chemical vapor deposition (CVD) using a single source precursor to increase its density, while maintaining a high surface area and stability of the materials. The diameter of the carbon/TiO2 spheres could be controlled from 2 μm to 200 nm by varying the flow rate of nitrogen. TEM analysis revealed that a fraction of the spheres exhibited a core–shell structure, with a faint carbon shell surrounding the TiO2 sphere. The density of the carbon/TiO2structures was 0.70 g/mL, which is four times higher than that of pure carbon nanotubes and spheres synthesized by CVD.
- Type
- Articles
- Information
- Journal of Materials Research , Volume 28 , Issue 3: Focus Issue: Titanium Dioxide Nanomaterials , 14 February 2013 , pp. 440 - 448
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 4
- Cited by




