Article contents
Synthesis and photocatalytic performance of SnZn(OH)6 with different morphologies
Published online by Cambridge University Press: 19 June 2013
Abstract
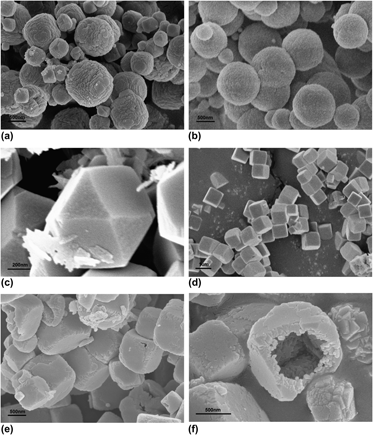
SnZn(OH)6 (ZHS) with different morphologies were synthesized by a facile self-templated method at room temperature. It was found that the morphology of ZHS could be controlled by varying the concentration of OH−. The crystalline structure and morphology of the particles were characterized by x-ray diffraction, UV-vis diffuse reflectance spectroscopy, scanning electron microscopy images, and N2 adsorption. The results indicated that the particles had almost uniform monoclinic geometry and uniform size. The photocatalytic activity of ZHS with different morphologies was tested through degradation of Rhodamine B (RhB). Therein, the hollow ZHS showed the highest light catalytic performance due to its large BET surface area, wide band gap, and high crystallinity.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2013
References
REFERENCES
- 6
- Cited by




