No CrossRef data available.
Article contents
Surfactant-assisted reflux synthesis of PbS nanostructures and their properties
Published online by Cambridge University Press: 29 October 2012
Abstract
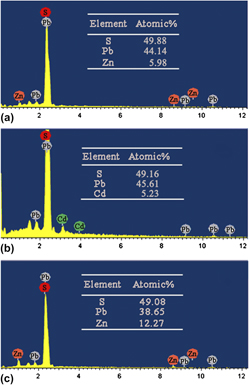
Uniform PbS nanostructures with varied morphology have been synthesized by a surfactant-assisted reflux route. ZnS and CdS layers were successfully coated onto PbS nanocrystals by encapsulation or epitaxial growth. The nanocrystals were characterized by x-ray diffraction, (high-resolution) transmission electron microscopy, selected area electron diffraction, and scanning electron microscopy. The truncated cubic nanostructures displayed a symmetric emission band at about 860 nm. Diffuse reflectance infrared (IR) spectroscopy was measured to estimate the band gap. High temperature and high frequency measurements of impedance and permittivity taught that the samples were stable and showed collateral evidence of the existence of epitaxial layers. Measurements illustrate that the luminescent properties of semiconductor PbS nanostructures are closely related to their surface nature, and encapsulation can affect their electrical properties and photoluminescence performance greatly. The study may prove useful in developing high frequency IR sensors and light signal amplification devices.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2012




