Article contents
Surface darkening phenomenon of Zn–Mg alloy coated steel exposed to aqueous environment at high temperature
Published online by Cambridge University Press: 09 December 2015
Abstract
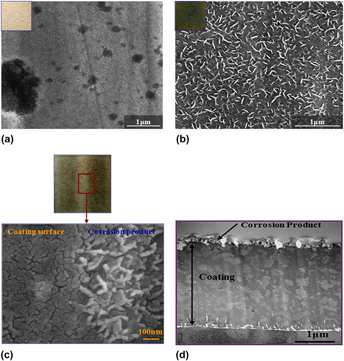
Steel coils coated with Zn–Mg alloy containing high Mg content develop dark rust when exposed to an extremely limited amount of aqueous environment. To understand the nature of the dark rust and its formation mechanism, the steel is evaluated by the immersion test and high temperature–humidity test followed by critical evaluation with transmission electron microscopy for cross-sectional observation, field-emission scanning electron microscopy for surface morphology observation, Auger electron spectroscopy and glow discharge spectroscopy for identification of chemical composition as a function of depth. The results indicate that the dark rust is formed by precipitation of Mg-based corrosion product on the outermost surface when the steel is exposed to aqueous environment at high temperature. This is due mainly to preferential dissolution of Mg phases by the galvanic action with MgZn2 and Mg2Zn11 composed of the coating layer, and easy precipitation of Mg2+ ion in a form of Mg(OH)2 in a limited volume of the condensed water film on the surface.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2015
References
REFERENCES
- 7
- Cited by




