Article contents
Supercapacitance properties of porous carbon from chemical blending of phenolic resin and aliphatic dicarboxylic acids
Published online by Cambridge University Press: 24 May 2016
Abstract
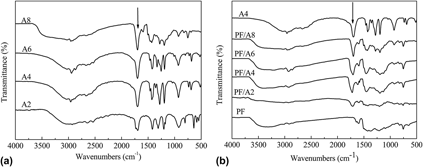
We have reported the chemical blending carbonization method to obtain microporous carbon with high surface area for application as electrode materials in supercapacitors. Aliphatic dicarboxylic acids with different methylene numbers (n = 2, 4, 6, and 8) react with phenolic resin (PF) during curing process. Abundant micropores are created in the carbon matrix after the decomposition of grafted or blocked diacids at temperature higher than 400 °C. The specific surface area (SSA) of the carbonized blending system increases with the diacid chain length, but decreases after n > 4 of the chain length. The maximum SSA of the blending system is up to 605.9 m2/g, which increased approximately 68% compared to that of the neat carbonized PF. Electrochemical investigation indicates that the highest specific capacitances of the blending system reaches 175 F/g at a specific current of 0.1 A/g in 30 wt% KOH aqueous electrolyte. Furthermore, the capacitance maintenance achieves 82.8% as the current density enlarged 55 times.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2016
References
REFERENCES
- 3
- Cited by





