Article contents
Structure–processing relationships of freeze-cast iron foams fabricated with various solidification rates and post-casting heat treatment
Published online by Cambridge University Press: 20 July 2020
Abstract
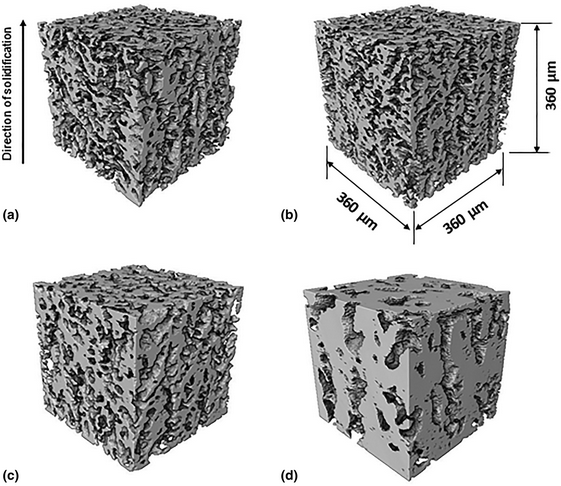
Iron foams are potential materials for the production, purification, and recuperation of hydrogen through redox systems. They are inexpensive, recyclable, and environmentally friendly. Nevertheless, iron foams cannot be employed repeatedly for redox cycling at high temperatures because the structure suffers morphological changes and a decrease in the effective porosity. In this work, two different pore structures of Fe-foams fabricated by freeze-casting have been produced: constant (CP) and gradient (GP) pore size. CP Fe-foams were obtained by employing a double-sided cooling technique to minimize gradients in pore width that result when using one-sided, constant cooling solidification techniques. GP Fe-foams were manufactured using a fixed-temperature cold plate. Optical microscopy and X-ray tomography were employed to characterize the pore structure and, for GP Fe-foams, to investigate the effect of redox cycling. After redox cycling, GP Fe-foams exhibited significant pore degradation.
Keywords
- Type
- Article
- Information
- Journal of Materials Research , Volume 35 , Issue 19: Focus Issue: Porous Metals: From Nano to Macro , 14 October 2020 , pp. 2587 - 2596
- Copyright
- Copyright © Materials Research Society 2020
References
- 8
- Cited by





