Article contents
Solid state synthesis and characterization of n–p (SnO2)1.3/(α ∼ Bi2O3)x/(β ∼ Bi2O3)1−x photocatalyst modulated by PVA and its photocatalytic performance
Published online by Cambridge University Press: 28 May 2019
Abstract
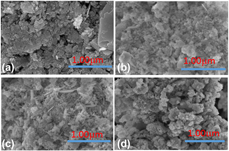
A kind of n–p (SnO2)1.3/(α ∼ Bi2O3)x/(β ∼ Bi2O3)1−x nanocomposite (SB-15) was synthesized with polyvinyl alcohol (PVA) as a template by solid state synthesis. XRD and HR-TEM confirmed the formation of n–p (SnO2)1.3/(α ∼ Bi2O3)x/(β ∼ Bi2O3)1−x. Particle size is found to be about 18 nm from HR-TEM images. FE-SEM clearly detected the boundary between SnO2 nanoparticles and Bi2O3 polyhedron particles. The special morphology and coexisting of α-Bi2O3 and β-Bi2O3 in SB-15 make it have a stronger visible light absorption range as far as 725 nm. PL and photocurrent test shows that the SB-15 has the best photocarriers separation capability. About 99% decolorization ratio of Rh.B was achieved in only 5 min. About 70% Cr6+ was degraded within 20 min and it is about 60% for tetracycline in the coexisting system (Te with Cr6+ solution), introducing it as a promising photocatalytic material. This work has addressed the method of phase-selective synthesis of n–p SnO2/α ∼ Bi2O3/β ∼ Bi2O3 by convenient solid state synthesis, which should be useful for the studies of other composites.
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2019
References
- 2
- Cited by




