Article contents
Sol–gel derived silica/polyethylene glycol hybrids as potential oligonucleotide vectors
Published online by Cambridge University Press: 28 November 2019
Abstract
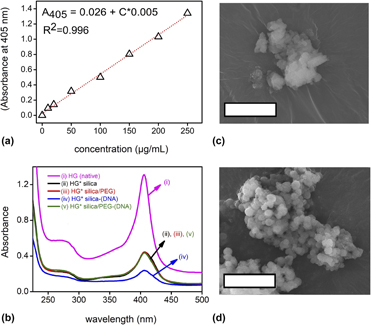
Until recently, mesoporous silica (MPS) particles have been successfully used in various biomedical applications including drug delivery. In the past decades, the research on MPS shifted sharply to gene delivery owing to its biocompatible, mesoporous structure that allows for loading oligonucleotides, shielding in the bloodstream, and delivering them to patient cells’ cytoplasm to stop cells’ genetic transcription. Until now, researchers faced several unique challenges and MPS, as oligonucleotide vectors, could not reach the clinical stage. In this study, material-related challenges were endeavored to overcome by a combined particle synthesis/oligo-loading strategy. DNA-encapsulated silica/polyethylene glycol (PEG) hybrid xerogels were synthesized at one step, via sol–gel technique. The xerogels were grinded into particles and characterized by X-ray diffraction, scanning electron microscopy, ultraviolet–visible spectroscopy, Fourier transform infrared spectroscopy, and gas adsorption analysis. The results demonstrated that uniform oligo-loaded silica/PEG hybrid xerogels could be synthesized without surface modification. Oligonucleotides were encapsulated inside the whole porous network, rather than attached only to particle surfaces as such in the conventional route. The results showed that PEG incorporation led to formation of monolithic xerogels, which could be grinded into spherical particles (557 ± 110 nm) with well-defined edges. Due to grinding, PEG chains were present both in the interior and on the surface of the particles. 10% PEG incorporation into silica precursor (tetraethyl orthosilicate) increased the resistance of DNA-encapsulated silica against protein degradation. In the overall sol–gel-derived silica/PEG hybrid materials were revealed as potential candidates for gene delivery applications such as RNA interference therapies.
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2019
References
- 12
- Cited by




