Article contents
Si-doped high-energy Li1.2Mn0.54Ni0.13Co0.13O2 cathode with improved capacity for lithium-ion batteries
Published online by Cambridge University Press: 07 December 2018
Abstract
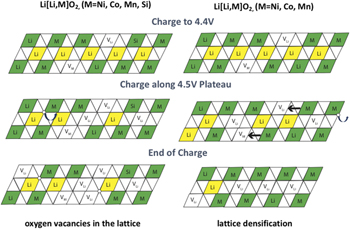
Li[Lix/3Mn2x/3M1−x]O2 (M = Ni, Mn, Co) (HE-NMC) materials, which can be expressed as a combination of trigonal LiTMO2 (TM = transition metal) and monoclinic Li2MnO3 phases, are of great interest as high capacity cathodes for lithium-ion batteries. However, structural stability prevents their commercial adoption. To address this, Si doping was applied, resulting in improved stability. Raman and differential capacity analyses suggest that silicon doping improves the structural stability during electrochemical cycling. Furthermore, the doped material exhibits a 10% higher capacity relative to the control. The superior capacity likely results from the increased lattice parameters as determined by X-ray diffraction (XRD) and the lower resistance during the first cycle found by impedance and direct current resistance (DCR) measurements. Density functional theory (DFT) predictions suggest that the observed lattice expansion is an indication of increased oxygen vacancy concentration and may be due to the Si doping.
Keywords
- Type
- Invited Paper
- Information
- Copyright
- Copyright © Materials Research Society 2018
References
REFERENCES
- 9
- Cited by




