Article contents
Preparation and characterization of glass–ceramic reinforced alginate scaffolds for bone tissue engineering
Published online by Cambridge University Press: 28 November 2019
Abstract
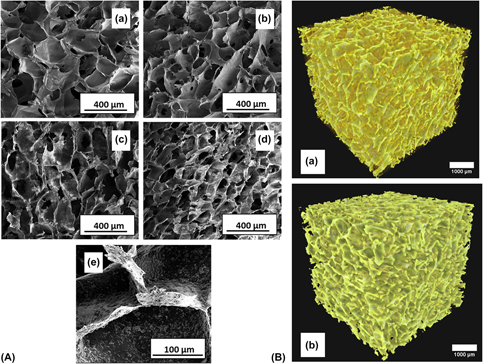
Bioactive glass–ceramic powder reinforced alginate scaffold has been successfully prepared and characterized for bone tissue engineering application. Glass-ceramic (GC) particles were synthesized through a sol–gel process. Alginate scaffolds containing different weight percentages of GC were fabricated through a freeze-drying technique. The composite scaffolds were characterized for phase analysis through X-ray powder diffraction and microstructure analysis through field emission scanning electron microscopy. The swelling behavior, degradation behavior, bioactivity, cell adhesion, and osteogenic potential of the fabricated scaffolds were evaluated. Microstructural analysis showed a highly porous behavior of the scaffold having a macroporous pore size. The composite scaffolds showed good bioactivity where GC induces apatite formation. The compressive strength of the scaffold was enhanced with GC addition due to the reinforcement of the alginate matrix. In vitro cell studies revealed that the composite scaffolds promoted cell adhesion, proliferation, and osteogenesis. Fabricated scaffolds are a promising biomaterial candidate for bone substitution because of their attractive properties.
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2019
References
- 10
- Cited by




