Article contents
New buffer layer material La(Pr)CrO3 for intermediate temperature solid oxide fuel cell using LaGaO3-based electrolyte film
Published online by Cambridge University Press: 15 June 2012
Abstract
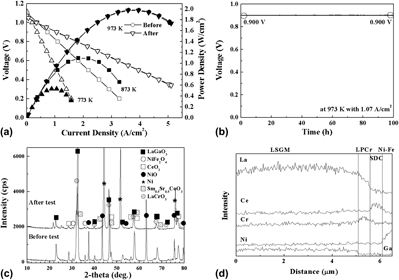
A metal-supported solid oxide fuel cell (SOFC) using Ce0.8Sm0.2O2 (Sm-doped ceria, SDC) buffer layer and La0.9Sr0.1Ga0.8Mg0.2O3 (LSGM) electrolyte films showed a small degradation in the cell performance after a long-term operation because of La migration from the electrolyte to the buffer layer, resulted in a formation of a less conductive phase. Thus, various ceramic materials such as doped ceria and perovskite-related oxides were investigated for an effective buffer layer with respect to fabricating reliable metal-supported SOFCs using a LSGM electrolyte film. In particular, La-doped CeO2 (LDC) and Pr-doped LaCrO3 (LPCr) were investigated as buffer layer material since the materials showed chemical compatibility with the LSGM and anode materials. The cell using a LDC buffer layer showed a prior stability during the operation for 100 h at 973 K, while the power density of the cell was slightly low owing to the low electrical conductivity of LDC compared with that of SDC or LPCr. In contrast, the cell using a LPCr buffer layer revealed significantly low open circuit voltage (OCV) and power density, which were attributed to Pr decomposition in the LPCr caused by the reactivity with water vapor. However, the metal-supported cell with a multilayer electrolyte film including LSGM/LPCr/SDC layers showed an almost theoretical OCV and reasonably high power density with no degradation after a long-term operation for 100 h at 973 K, suggesting that the LPCr layer effectively prevented La migration and the SDC layer led to avoid the Pr decomposition. Thus, a LPCr is an effective buffer layer material for reliable metal-supported SOFCs using a LSGM electrolyte thin film.
- Type
- Articles
- Information
- Journal of Materials Research , Volume 27 , Issue 15: Focus Issue: Advanced Materials for Fuel Cells , 14 August 2012 , pp. 1906 - 1914
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 5
- Cited by




