Article contents
Near-zero thermal expansion and phase transition in In0.5(ZrMg)0.75Mo3O12
Published online by Cambridge University Press: 27 September 2016
Abstract
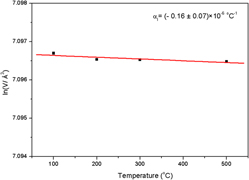
Physical properties of In0.5(ZrMg)0.75Mo3O12, including the coefficient of thermal expansion, phase stability, hygroscopicity, and decomposition temperature have been thoroughly studied by in situ x-ray powder diffraction, Raman spectroscopy and thermal methods. These investigations show that In0.5(ZrMg)0.75Mo3O12 exists in a monoclinic phase (P21/a) at room temperature and transforms to an orthorhombic (Pbcn) phase at ∼82 °C. In the orthorhombic form this material presents intrinsic near-zero thermal expansion (−0.16 × 10−6 K−1) in the range between 100 and 500 °C. The phase is not hygroscopic, but starts to decompose into its constituent oxides at temperatures higher than 700 °C. In comparison to the end member phase ZrMgMo3O12 in the In2Mo3O12–ZrMgMo3O12 solid solution, In0.5(ZrMg)0.75Mo3O12 is less promising for near room-temperature applications due to the phase transition from monoclinic to orthorhombic slightly above room temperature. However, the orthorhombic phase of In0.5(ZrMg)0.75Mo3O12 has potential for applications that require zero thermal expansion at temperatures higher than 100 °C.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2016
References
REFERENCES
- 10
- Cited by





