Article contents
Nanostructured lipid carrier delivering chlorins e6 as in situ dendritic cell vaccine for immunotherapy of gastric cancer
Published online by Cambridge University Press: 03 November 2020
Abstract
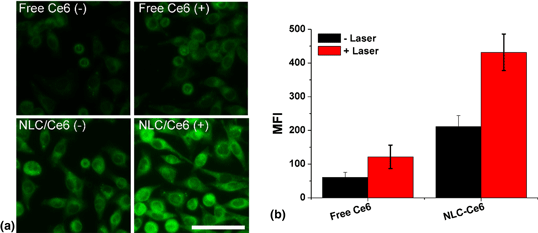
The recent scientific progress has shown the promising effect of the vaccine in immunotherapy of cancer, which relies on the antigen processing/presentation capability of dendritic cells (DCs). As a result, cancer vaccines targeting DC, which also named as DC vaccine, was a hot-spot in vaccine development. Herein, a nanostructured lipid carrier (NLC) was employed to load chlorin e6 (Ce6) to serve as a potential in situ DC vaccine (NLC/Ce6) for effective immunotherapy of gastric cancer. Taking advantage of the photodynamic effect of Ce6 to generate reactive oxygen species (ROS) under laser irradiation, the NLC/Ce6 was able to trigger cell death and expose tumor-associated antigen (TAA). Moreover, mimicking the natural inflammatory response, the ROS can also recruit the DC for the effective processing/presentation of the in situ exposed TAA. As expected, we observed strong capability DC vaccination efficacy of this platform to effectively inhibit the growth of both primary and distant gastric tumors.
- Type
- Article
- Information
- Copyright
- Copyright © The Author(s), 2020, published on behalf of Materials Research Society by Cambridge University Press
Footnotes
These authors contributed equally to this work.
References
- 6
- Cited by





