Article contents
Multimineral nutritional supplements in a nano-CaO matrix
Published online by Cambridge University Press: 03 April 2013
Abstract
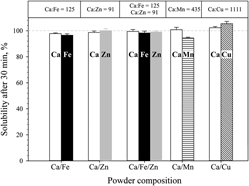
The fast dissolution of certain calcium-containing compounds makes them attractive carriers for trace minerals in nutritional applications, e.g., iron and zinc to alleviate mineral deficiencies in affected people. Here, CaO-based nanostructured mixed oxides containing nutritionally relevant amounts of Fe, Zn, Cu, and Mn were produced by one-step flame spray pyrolysis. The compounds were characterized by nitrogen adsorption, x-ray diffraction, (scanning) transmission electron microscopy, and thermogravimetric analysis. Dissolution in dilute acid (i.d.a.) was measured as an indicator of their in vivo bioavailability. High contents of calcium resulted in matrix encapsulation of iron and zinc preventing formation of poorly soluble oxides. For 3.6 ≤ Ca:Fe ≤ 10.8, Ca2Fe2O5 coexisted with CaO. For Ca/Zn compounds, no mixed oxides were obtained, indicating that the Ca/Zn composition can be tuned without affecting their solubility i.d.a. Aging under ambient conditions up to 225 days transformed CaO to CaCO3 without affecting iron solubility i.d.a. Furthermore, Cu and Mn could be readily incorporated in the nanostructured CaO matrix. All such compounds dissolved rapidly and completely i.d.a., suggesting good in vivo bioavailability.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2013
References
REFERENCES
- 5
- Cited by




