Article contents
Low-temperature performance of electrochemical capacitors using acetonitrile/methyl formate electrolytes and activated carbon fabric electrodes
Published online by Cambridge University Press: 20 January 2020
Abstract
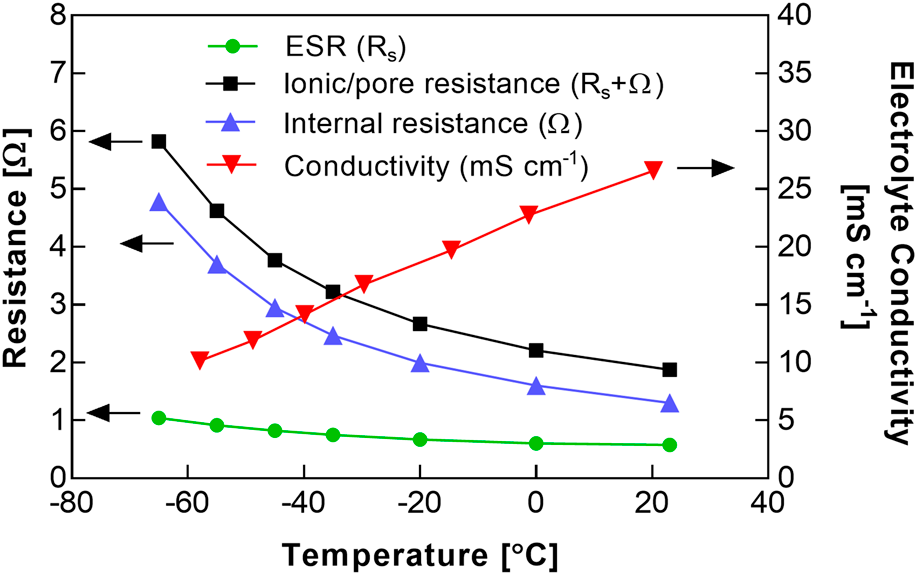
Electrochemical capacitors featuring a modified acetonitrile (AN) electrolyte and a binder-free, activated carbon fabric electrode material were assembled and tested at <−40 °C. The melting point of the electrolyte was depressed relative to the standard pure AN solvent through the use of a methyl formate cosolvent, to enable operation at temperatures lower than the rated limit of typical commercial cells (−40 °C). Based on earlier electrolyte formulation studies, a 1:1 ratio of methyl formate to AN (by volume) was selected, to maximize freezing point depression while maintaining a sufficient salt solubility. The salt spiro-(1,1′)-bipyrrolidinium tetrafluoroborate was used, based on its improved conductivity at low temperatures, relative to linear alkyl ammonium salts. The carbon fabric electrode supported a relatively high rate capability at temperatures as low as −65 °C with a modest increase in cell resistance at this reduced temperature. The capacitance was only weakly dependent on temperature, with a specific capacitance of ∼110 F/g.
Keywords
- Type
- Invited Paper
- Information
- Copyright
- Copyright © Materials Research Society 2020
References
- 10
- Cited by





