Article contents
Low density ceramic foams from alumina-sucrose using magnesium nitrate as a blowing and setting agent
Published online by Cambridge University Press: 09 September 2016
Abstract
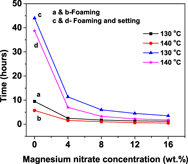
The effect of magnesium nitrate on the thermo-foaming of powder dispersions in molten sucrose for the preparation of alumina foams has been studied. The magnesium nitrate decreases the melting point of sucrose from 180 to 160 °C and acts as a blowing and setting agent. The foaming time and setting time decreases with an increase in foaming temperature as well as an increase in magnesium nitrate concentration. The rapid foaming followed by foam collapse is observed beyond 140 °C. The accelerated foaming and setting is due to an increase in the rate of –OH condensation because of the catalytic effect of H+ generated by the hydrolysis of magnesium nitrate. The porosity of alumina foam increases while cell size and grain size decrease with an increase in magnesium nitrate concentration. A change in foam structure, from partially interconnected cellular to completely interconnected reticulate-like, occurs when the magnesium nitrate concentration increase from 4 to 8 wt%.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2016
References
REFERENCES
- 2
- Cited by





