Article contents
Facile preparation of macro-mesoporous zirconium titanate monoliths via a sol–gel reaction accompanied by phase separation
Published online by Cambridge University Press: 30 September 2019
Abstract
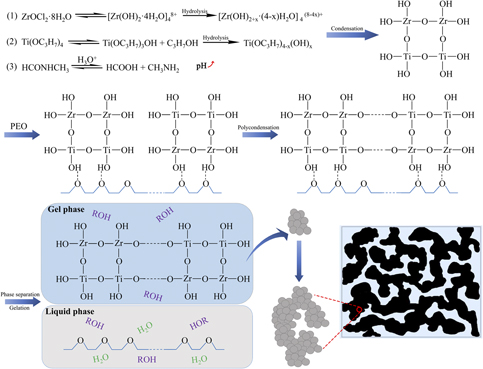
Macro-mesoporous zirconium titanate monoliths have been successfully prepared via a sol–gel process accompanied by phase separation, with poly(ethylene oxide) (PEO) as a phase separation inducer and N-methylformamide (NFA) as a gelation accelerator. The size and morphology of macropores are controlled by the PEO and NFA amount. By choosing appropriate starting composition, the co-continuous structure could be obtained. The as-dried gel is amorphous, the pore size is in the range of 0.05–0.5 μm, the porosity is 46%, and the surface area is 111 m2/g. After heat treatment at 500 °C, the gel transforms into the phase ZrTiO4, the macropore diameter decreases slightly, the porosity increases to 63%, and the surface area decreases to 40 m2/g. Moreover, the macroporous structure is well maintained, and the skeleton becomes dense and smooth. The samples have macropores, mesopores, and micropores before and after heat treatment.
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2019
References
- 6
- Cited by




